1. INTRODUCTION
High purity lithium hydroxide (LiOH) is a widely used industrial chemical, in catalysts [1,2] and as a carbon dioxide absorbent [3,4]. In particular, it is used as a positively charged electrode material and as an electrolyte in lithium ion batteries [5,6]. Due to the rapid increase in the usage of Li-containing products in electric vehicles, a substantially equivalent amount of lithium waste is also produced. Numerous attempts have been made to recycle lithium waste, which has resulted in the recovery of lithium precursors [7-10]. However, the recovered Li contains other impurities such as magnesium, calcium, and nickel, which makes it difficult to synthesize high purity LiOH. A synthetic method of recovering Li from recycled lithium precursors would be highly valuable.
Among the various methods that have been studied to synthesize LiOH, ion exchange and electrolysis have been the most commonly used due to their simplicity [11-16]. However, an ion exchange method using simple precipitation is preferable to electrolysis, if it could be accomplished using specific equipment such as an exchange membrane and result in high purity.
LiOH is typically synthesized using a lithium carbonate (Li2CO3) precursor. To date, few studies have employed lithium sulfate (Li2SO4) for the same process. Because Li2CO3 has low solubility (1.54 g/100 g, 0 °C water) its conversion efficiency into LiOH is less than 60% in water, as reported in numerous papers. This is because Li2CO3 only reacts with a limited surface area by dissolution and ion exchange, and as a result, a mixed phase of Li2CO3and LiOH co-exist in one solution [10].
In this study, we synthesized LiOH nanoparticles using recycled Li2SO4 from spent discarded Li-containing products with Ba(OH)2. To enhance productivity, highly soluble Li2SO4 (26.1 g/100 g, 0 °C water) and a barium hydroxide (Ba(OH)2) precursor were chosen to prepare LiOH via a twostep precipitation method. We were able to achieve the complete conversion of the precursors into LiOH through the two-step precipitation method. Ba(OH)2 was selected due to its insolubility with Ba(SO)4, which was a byproduct, in water. The solution was evaporated to obtain LiOH powder, which in turn was used to analyze crystallinity and morphology. Precursor ratio, reaction time, reaction temperature and drying temperature were all evaluated to optimize the reaction conditions.
2. EXPERIMENTAL PROCEDURE
Initially, 0.01 mol of recycled Li2SO4 (Sungil Co., 99%) precursor was put into 100 ml of D.I water at room temperature (RT) and stirred at 300 rpm for 20 min to be fully dissolved. Subsequently, the solution was heated between RT and 60 °C at an increasing speed of 5 °C/min. When the Li2SO4 precursor reached the set temperature value, 0.01 ~ 0.05 mol of Ba(OH)2 (Sigma Aldrich Co. 95%) was added to the solution and reacted for 2 to 6 hours. After the reaction, the precipitates were removed, and the solution was evaporated to collect powder. For the complete conversion of the precursors, a two-step precipitation method was employed by adding equal amounts of D.I water, Li2SO4, and half of the above-mentioned Ba(OH)2 into the solution at 40 °C for 1 h. The powders that precipitated were filtered and removed while the solution was left to react with the remaining Ba(OH)2 at the same conditions. After the reaction, the solution was filtered with a 450 nm microfilter. The filtered solution was further evaporated using a hot plate at a solution temperature between 70 ~ 90 °C, until a white powder started to emerge.
In order to examine its crystal structure and primary particle size, the powder was pressed into a certain shape and analyzed by X-ray diffraction (XRD, Hitachi, Japan) with Cu Kα (λ=1.54 Å) radiation at a scanning speed of 5 °/min. The morphologies of the synthesized nanoparticles were analyzed by field emission scanning electron microscope (FE-SEM, JEOL, Japan); the crystallinity was evaluated with selected area diffraction (SAED) and its individual particle sizes were confirmed by transmission electron microscope (TEM, JEOL, Japan). Furthermore, the powder was dispersed in ethanol and sonicated for 20 min; subsequently, it was dropped onto a Cu grid and dried in a chamber at 80 °C for 24 hours.
3. RESULTS AND DISCUSSION
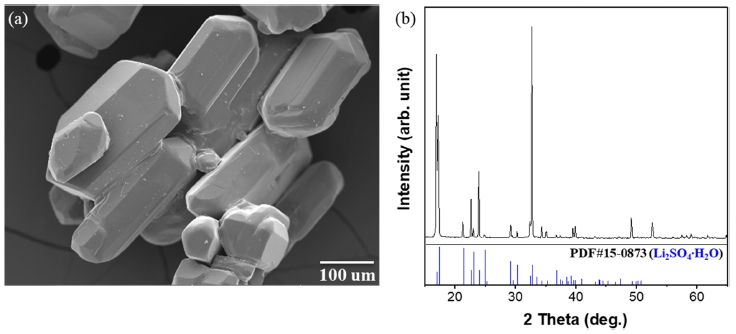
As shown in Fig 1 (a), SEM and XRD were performed to analyze the initially recycled Li2SO4 precursor obtained from the Li-containing spent battery. According to the SEM image, the initial morphology of the Li2SO4 was a hexagonal prism structure with length in the range of 80.1 ~ 297 μm and width in the range of 78.1 ~ 164 μm. The XRD pattern in Fig 1 (b) identifies that it has a monoclinic Li2SO4 structure in accordance with the JCPDS 15-0873.
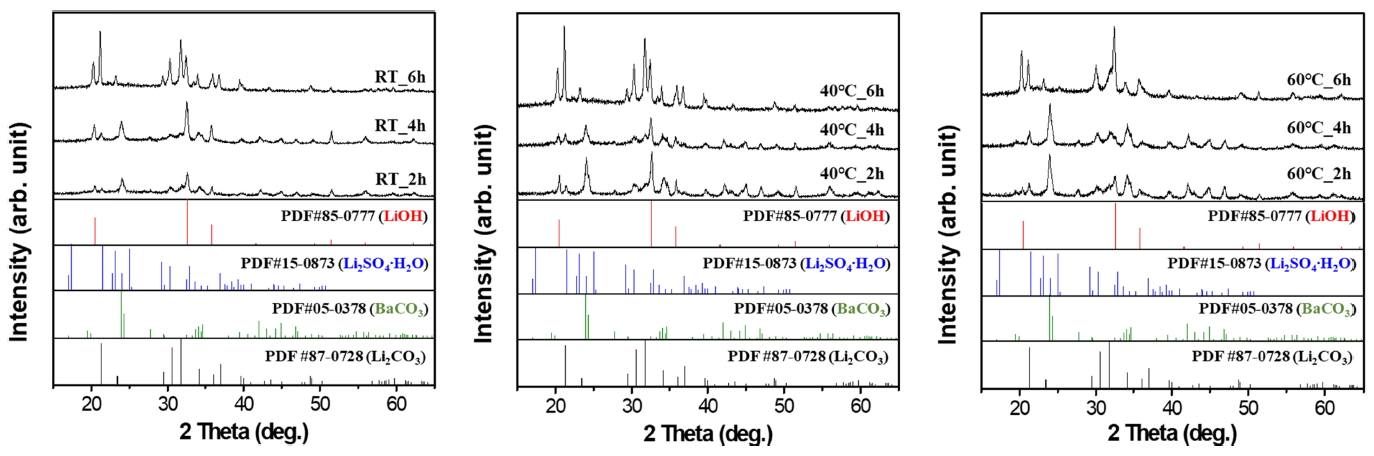
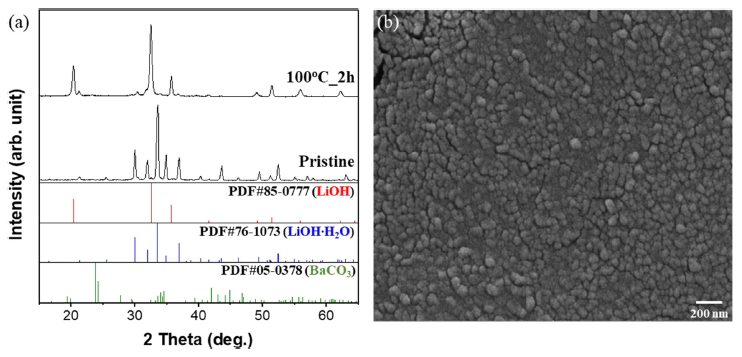
Various parameters were considered and tested to optimize the reaction conditions between LiOH, which was synthesized using 0.01 mol Li2SO4 with 0.01 mol Ba(OH)2. These parameters were reaction temperature and time, as shown in Fig 2 XRD. Fig 2 (a) shows patterns of synthesized powders with different reaction times at RT.
Li2CO3 is known to have a lower solubility in water and reacts through an ion exchange mechanism at the limited surface area. However, other precursors that have a high solubility trigger the nucleation and growth mechanism; this leads to their complete conversion into a different crystal structure and morphology, as compared to the initial precursor [9,17]. Following this reaction, the hydrate precursor of Li2SO4 was completely transformed. A 2hour reaction at RT showed a low intensity of the LiOH and BaCO3 mixed phase. As the reaction time increased, not only did the LiOH peak increase, but Li2CO3 also newly emerged. LiOH can easily be transformed into Li2CO3 when it is heated above 100 °C in ambient air due to its characteristic nature of absorbing CO2 [19].
In the case of BaCO3, synthesized BaSO4 was removed by filtration following the reaction equation; this was due to its low solubility in water, which indicated that the unreacted initial Ba(OH)2 precursor was transformed. Characteristics similar to that of LiOH were also observed in Ba(OH)2. It could be easily observed reacting with CO2 in air when it was heated between 40 ~ 90 °C [18]. Fig 2 (b) shows the reaction at 40 °C with different reaction periods. Even though the reaction time was short, it was observed that LiOH was synthesized with high intensity. However, as the time increased, it also transformed into Li2CO3, showing a tendency similar to that observed under RT conditions.
At the reaction temperature of 60 °C, a lower peak of LiOH was observed and Li2CO3 emerged at an earlier stage. This can be explained by the fact that the conversion efficiency of the precursor significantly depends on the reaction temperature. The precipitation process with higher temperature promotes faster particle growth synthesis. This is due to increasingly active ions in solutions with more highly mobile ionized precursors. Through a series of experiments, the optimum conditions for LiOH preparation was then determined to be 40 °C for a reaction time of 2 hours.
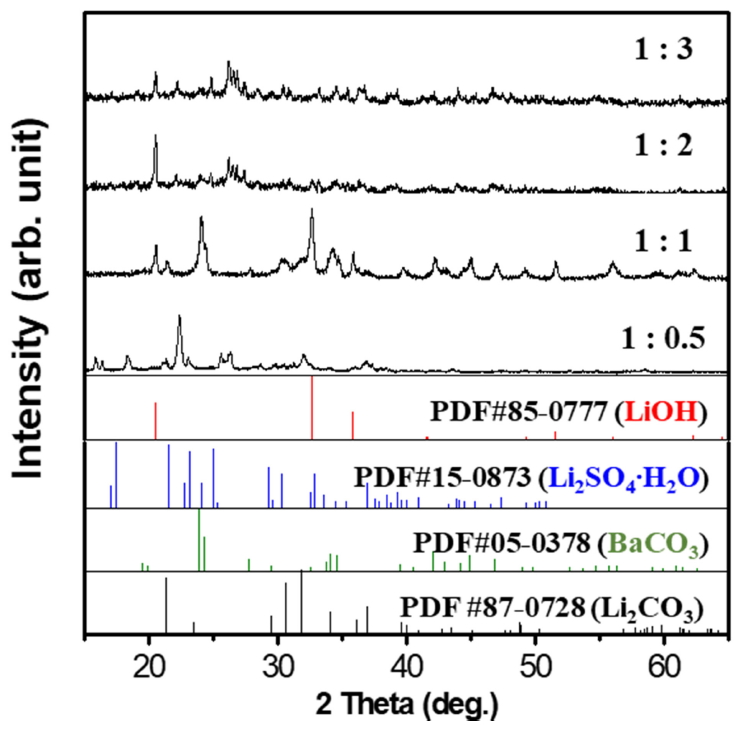
In order to understand the effect of precursor concentration on the synthesis of LiOH, the Ba(OH)2 ratio was controlled between 1/0.5 ~ 1/3 when fixing the Li2SO4 concentration. Fig 3 shows the XRD patterns with different precursor ratios. According to this analysis, at a lower ratio Ba(OH)2 produced BaSO4, which was removed through filtration. However, the intermediate crystal structure of lithium hydroxide sulfate was not removed (3LiOH-Li2SO4), and was separately identified by JCPDS card no. 32-0598. When the Ba(OH)2 ratio increased above 1/2, LiOH was produced. Moreover, unreacted Ba(OH)2 with its high solubility penetrated the filter and remained in solution, which after evaporation showed a LiOH and Ba(OH)2 mixed crystal structure. Thus, for the optimum conditions, a precursor ratio of 1:1 was chosen.
Generally, the precipitation process is a one-step reaction that easily converts into the objective precipitant. However, the low conversion efficiency of the Li2SO4 precursor impedes this reaction, as previously shown in Fig 2. For Li2CO3, to completely transform the precursor into LiOH and enhance efficiency, we precipitated it through a two-step reaction. The optimum conditions for the synthesis were a precursor ratio of 1:1, a temperature of 40 °C and a 2 hour reaction condition.
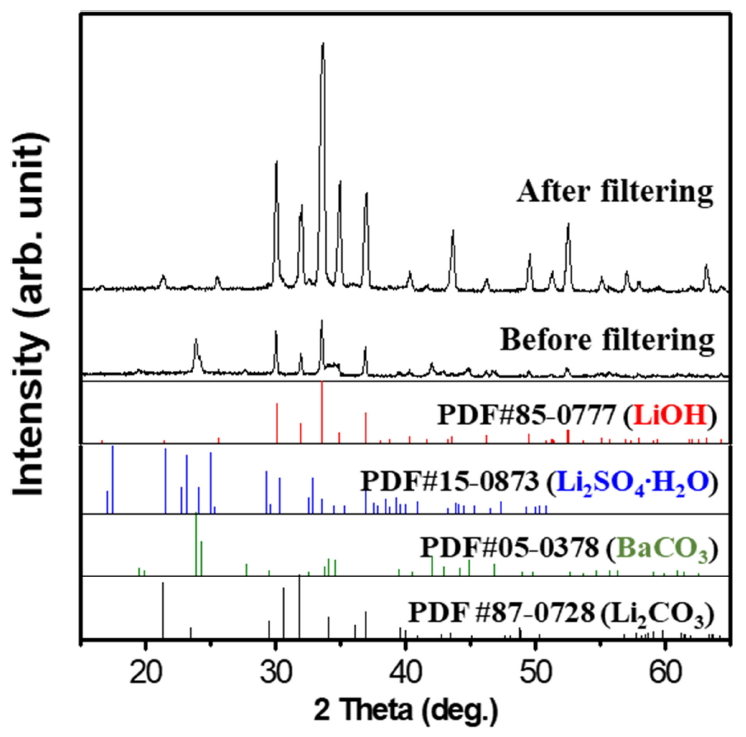
In Fig 4, the XRD results show the peak of the two-step synthesized precipitant after filtration. LiOH-H2O and BaCO3 co-exist in the precipitant, which also showed tendencies similar to the one-step precipitation method. However, the huge difference between the reactions is that insoluble BaCO3 and soluble LiOH-H2O are the primary ingredients, without any Li2CO3, which was observed in the one-step precipitant.
Accordingly, the synthesized powder was dissolved in D.I water and filtered again. According to the XRD data in Fig 4 after filtering LiOH-H2O was finally produced without any impurities.
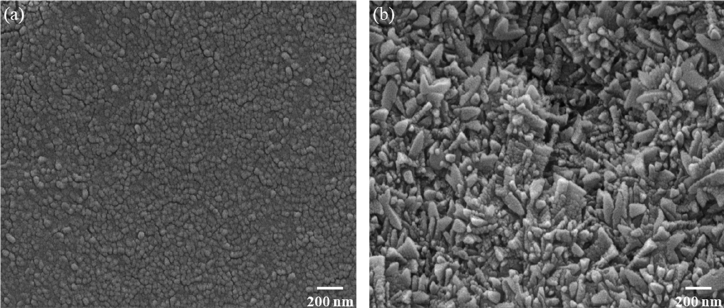
The morphology and crystallinity of the LiOH nanoparticles depends on the evaporation temperature, and this was investigated under various conditions between 70 ~ 90 °C using SEM analysis, as shown in Fig 5. According to the images in Fig 5 (a), at an evaporation temperature of 70 °C, uniformly distributed nanoparticles with a size of 35.8 ± 9.77 were formed following the slow nucleation and growth process. When the temperature increased to 90 °C, LiOH started to agglomerate and was grown with an irregularly mixed morphology of nanorods and pyramid-like structures with widths of 52.0 ± 12.4 and lengths of 194 ± 14.7, as can be seen in Fig 5 (b). Thus, the evaporation temperature of 70 °C was chosen as optimum, for its uniformity and high surface area.
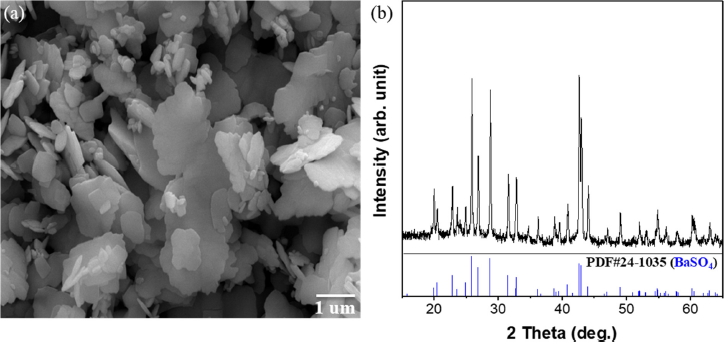
XRD and SEM analysis was conducted for the filtered precipitants after the two-step precipitation reaction, to determine conversion efficiency and the existence of other impurities. In the SEM image in Fig 6 (a), irregular sheets with some stacked structures can be seen. In the XRD analysis in Fig 6 (b), pure BaSO4 was produced (JCPDS card no. 24-1035) with no other impurities. This confirmed the optimum precursor ratio.
The as-prepared LiOH·H2O nanoparticles were thermally treated to remove any hydrates and were transformed at 100 °C for 2 hour in an atmosphere of N2 into LiOH. Above 100 °C, the annealing process promotes CO2 adsorption and LiOH easily converts into Li2CO3, which can be identified in Fig 2.
In Fig 7, the XRD pattern after calcination occurred reveals that the initial monoclinic phase of LiOH-H2O (JCPDS card no. 76-1037) transformed into a pure tetragonal structure of LiOH (JCPDS card no. 85-0777) with high crystallinity. By comparing the XRD peaks before and after calcination, no changes, such as broader or sharper phenomena, were observed. This means that the hydrate bond was removed by heat treatment without inducing any differences in the size of the crystallites of the two samples, leaving Li with a purity of 99.6%, as shown in Table 1 by ICP analysis.
TEM analysis was performed to identify the crystalline structure of the LiOH nanoparticles at total precursor ratio of 1:1 with two-step precipitation and an evaporation temperature of 70 °C. Fig 8 (a) shows low magnification images of the LiOH nanoparticles. They appear as regularly distributed nanoparticles in accordance with the SEM images in Fig 7. In the high magnification image in Fig 8 (b), individual primary particles were agglomerated into nanoparticles during the nucleation and growth process.
For further analysis, HRTEM, as seen in Fig 8 (c) was performed to elucidate the crystalline structure of the LiOH. Lattice spacings in the LiOH nanoparticles were found to be 0.22 nm and 0.15 nm, which correspond to the (310) and (002) planes in tetragonal LiOH, respectively [20]. The specific lattice constant of the LiOH nanoparticle was confirmed by the dot shaped SAED patterns of the (310), (002), (621) and (060) planes, which are consistent with a single crystalline nature.
4. CONCLUSIONS
In order to produce uniform LiOH nanoparticles with high conversion efficiency, a two-step precipitation method was used. For the reaction conditions, a specific precursor ratio of 1:1 was chosen, and the hydroxyl containing the precursor was divided into halves. First, a precursor ratio of 1:0.5 was chosen to react for 60 min at a solution temperature of 40 °C, and subsequently filtered to remove any precipitates. After filtration, half of its hydroxyl precursor was placed into the filtered solution and was reacted under the same conditions. After the reaction, the precipitated powder was filtered, and the solution was evaporated at the solution temperature of 70 °C until a white powder emerged. In order to break hydrate bonds in the LiOH, thermal treatment was performed at a low temperature of 100 °C to prevent phase transformation by the adsorption of CO2. Using this method, we synthesized uniform nanoparticles with mean sizes of 35.8 nm and a highly purified LiOH tetragonal phase having 99.6% purity.