1. 서 론
전해 동박은 대량생산이 가능하고, 압연박 대비 폭이 넓기 때문에 상대적으로 저렴한 가격, 우수한 열 전도성, 낮은 전기저항 등의 장점으로 인하여 노트북, 스마트폰, PC 등 휴대기기의 회로배선소재로 널리 사용되어 왔다[1-3]. 또한 구리는 뛰어난 부식 특성을 보여 식수나 산업용수의 파이프 라인으로 사용되고 있을 뿐만 아니라[4-6], 최근에는 최첨단 산업인 하이브리드 전기자동차(HEV), IT, 지능형 로봇산업, 친환경 에너지 산업 등의 발전에 따라 이들의 핵심 부품인 이차전지 음극 소재로 사용되고 있다[7].
전기도금법을 이용하여 전해동박의 제조는 첨가제, 음극 전압, 전류밀도, 전해액의 조성 등 다양한 도금 변수에 의하여 표면 형상과 결정구조 및 전기적 특성, 기계적 특성에 영향을 받는다[8-12]. 전기 도금법을 이용하여 다양한 두께의 전해 동박을 제조 할 경우 기본 조성을 변경해가며 생산하기에는 유지비용 및 공정처리 등의 어려움으로 인하여 기본 조성에 특정 첨가제 첨가를 통하여 원하는 물성을 맞추고 있다. 일반적으로 구리 전해 도금에 사용되는 첨가제는 가속제(accelerator, brightener), 감속제(suppressor, carrier), 그리고 표면 단차를 제거하여 평탄한 도금층을 형성하도록 도와주는 평활제(leveler) 등이 사용되며[13,14]. 가속제로는 SPS, MPSA, DPS, Thiourea 등의 유기 화합물이 사용되는 것으로 알려져 있으며[15-17], 감속제로는 PEG, 젤라틴, 콜라겐 등의 중합체 계열 유기 화합물이 대표적으로 사용된다[4,18]. 평탄제는 JGB(Janus Green B), PEI, HEC 등 화합물이 주로 사용된다[19,20]. 일반적으로 동박의 생산량을 증대시키기 위한 고전류밀도에 적용 가능한 첨가제의 역할은 중요하며, 이들 첨가제의 개발은 더욱 중요하다고 할 수 있다. 그러나 높은 전류밀도를 이용하는 연속생산 공정에서 첨가제가 구리의 핵생성 및 결정성장에 미치는 효과에 대한 메커니즘을 규명되지 않은 부분이 많다. 대표적 평활제 첨가제의 하나인 JGB의 경우 저전류밀도에서 연구한 결과가 대부분으로 Park 등[21]은 3 ASD의 낮은 전류밀도에서 JGB를 첨가한 경우 첨가량이 증가할수록 초기 면저항이 증가하고, 결정립 성장이 억제되어 미세한 결정구조를 보인다고 보고하였다. Lee 등[22]은 JGB 첨가량별로 억제효과를 측정 시 첨가량에 따라 억제효과가 다름을 확인하였고, 인가전류량이 일정량 이상 증가한 경우 평활제 역할이 감소한다고 보고하였다. 또한 Jeong [23]은 JGB 첨가제 첨가시 결정립 크기가 감소하고 비저항이 벌크 대비 높게 나타나며, 첨가량이 일정수준 이상 첨가된 경우에는 self-annealing 수준의 열처리에서는 재결정이 이루어지지 않는다고 보고하였다. 이와 같이 대부분의 연구들이 저전류 밀도에서 수행되었으며, JGB의 첨가량에 따른 기계적 특성과의 상관관계는 규명되지 않았다. 이에 본 연구에서는 고전류밀도에서 JGB 첨가량에 따른 초기 분극현상과 이러한 분극현상으로 인한 전착의 표면 특성 및 결정구조를 파악하였으며, 기계적 특성 및 전기적 특성에 대하여 분석하였다.
2. 실험방법
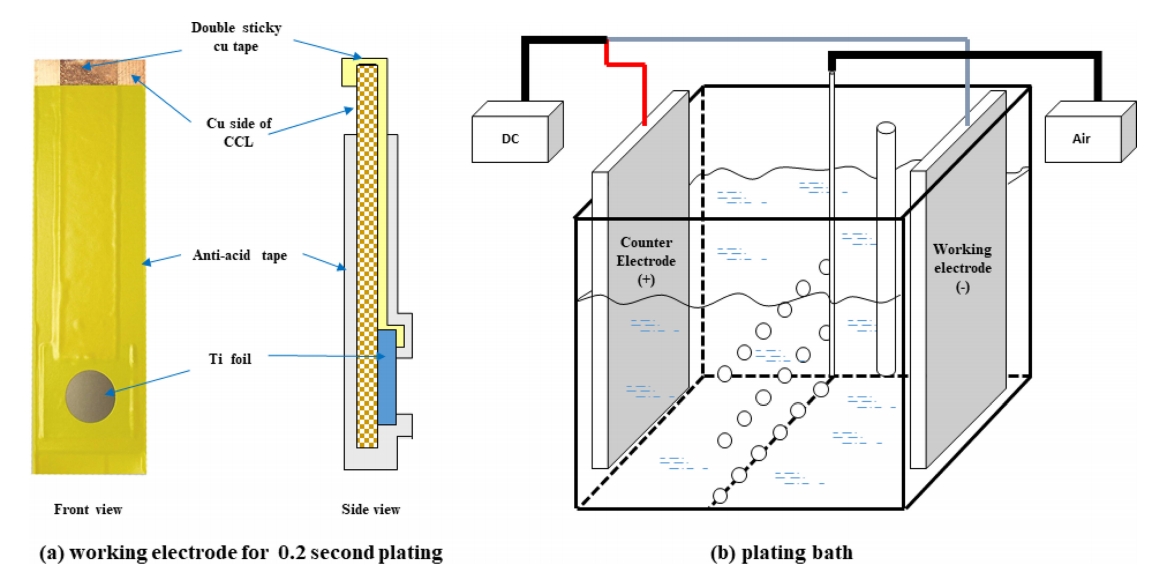
본 실험에서는 황산구리(CuSO4·5H2O), 황산(H2SO4), 염산 (HCl), 가속제인 MPSA (3-mercapto-1-propane sulfonic acid), 도금 억제제인 콜라겐(MW: 16,000~20,000)을 기본욕으로 하여 전해도금을 진행하였고 평활제로 JGB(Janus Green B)를 사용하였다. (표 1) 양극으로는 이산화이리듐 (IrO2)이 코팅된 불용성 양극을 사용하였으며, 음극으로는 티타늄판(99.9%)을 연마지를 이용하여 #1500까지 순차적으로 연마하여 사용하였다. 음극은 초기 핵생성 관찰용 샘플 제조를 위하여 20 mm × 20 mm 크기에서 1 cm2 면적이 노출되도록 하였고, 인장시험 샘플 제조를 위하여 50 mm × 150 mm 크기로 각각 다르게 제작하였다(그림 1). 극간 거리는 10 cm로 일정하게 유지하며 전류밀도 20 ASD 정전류 모드로 도금을 실시하였으며, 이 과정동안 도금액의 온도는 50 ℃(±0.5 ℃)로 일정하게 유지하였다. 초기 핵생성의 표면 형상을 관찰하기 위하여 0.2초 동안 도금을 실시하였으며, 물성분석을 위하여 도금층의 두께가 12 µm 되도록 도금을 실시하였다.
전착층의 표면형상 및 거칠기를 관찰하기 위하여 주사전자현미경(FESEM, SU-70, Hitachi, Japan) 및 표면조도측정기(SV-3000M4, Mitutoyo, Japan)를 각각 활용하였다. 결정크기 및 결정구조를 관찰하기 위하여 XRD(Dmax IIIA type, Rigaku Co., Japan)를 사용하였다. 4극 탐침 장치(CMT-SR1000N, AIT, Korea)를 사용하여 비저항을 측정하였고, 전착층의 두께를 확인하기 위하여 XRF(X-Starta 960, Oxford, U.S.A.)를 사용하였으며 16포인트를 측정하여 분석하였다. 구리 전착층을 타이타늄 판으로부터 분리하여 IPC-TM-650 규격에 맞게 12.7 mm × 100 mm 크기로 절단한 후 인장시험기(AG-X, Shimadzu, Japan)를 사용하여 인장강도와 연신율을 측정하였다. 각각의 측정된 분석결과는 Minitab 통계프로그램을 사용하여 통계 처리하였다.
3. 결과및고찰
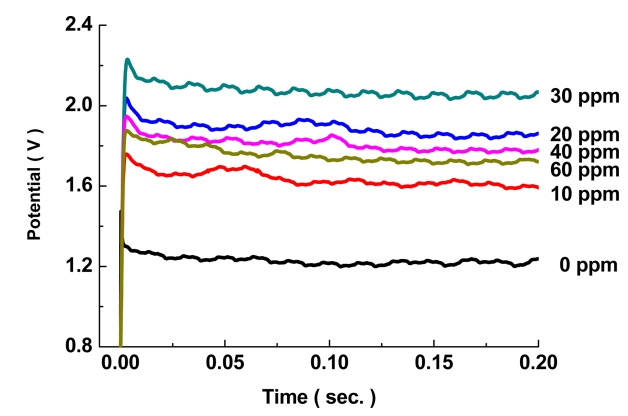
그림 2에서 JGB 첨가량을 0 ~ 60 ppm 첨가하여 초기 전압을 측정하였을 때, 무첨가군 대비 JGB 첨가군들에서 전체적으로 전압이 높게 나타나고 있다. 이러한 현상은 첨가제가 초기 생성된 구리결정에 흡착되어 결정성장을 억제하고 있기 때문이며, 이러한 분극현상은 30 ppm을 첨가한 경우에 가장 크게 나타나고 있으며, 40 ~ 60 ppm 조건에서는 오히려 분극현상이 감소하고 있다. Lee 등[22]에 따르면 저전류밀도(약 1.1 ASD)에서 JGB첨가제를 1 ~ 4 ppm 범위에서 첨가한 경우 1 ~ 3 ppm 범위에서 과전압이 증가한 후 3 ppm 이후 감소하는 현상보이고 있다. 이처럼 도금 조건에 따라 최대 과전압이 일어나는 첨가량은 다르나 일정 첨가량 이후 분극 현상이 감소하는 결과는 일치하였다. 이번 연구에서 30 ppm이후 분극현상이 감소한 결과는 0.2초간 도금된 표면을 관찰한 SEM 이미지를 통하여 변화를 관찰할 수 있다(그림 3).
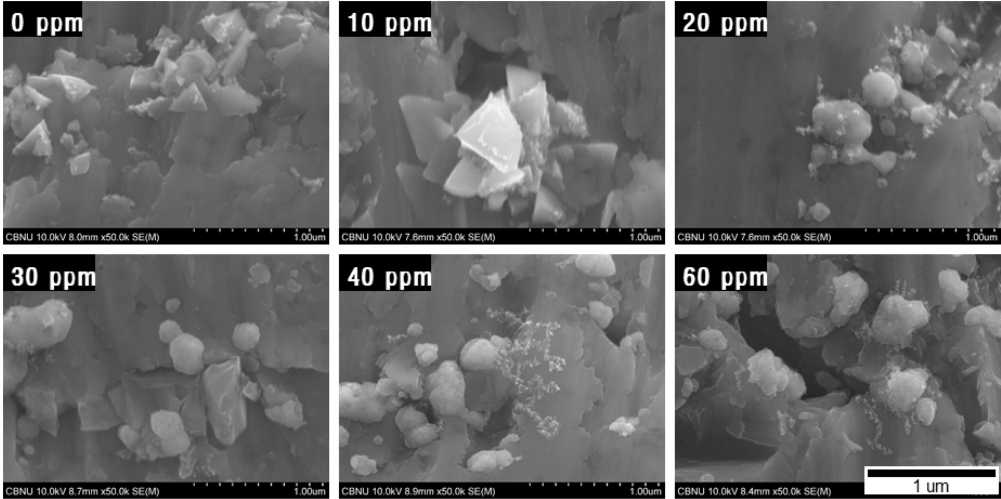
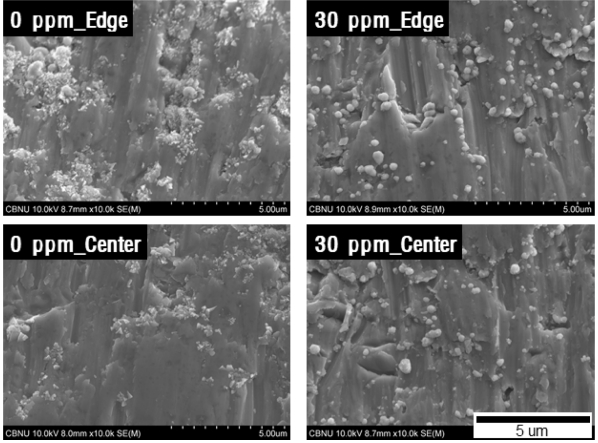
그림 3은 도금표면 중앙부를 관찰한 결과이며 첨가제를 첨가하지 않은 군과 JGB 첨가제를 10 ppm 첨가한 경우에는 각진 형상의 결정들이 관찰이 되었고, 20 ppm 이상의 조건에서는 구형형태의 결정들이 관찰되고 있다. JGB가 평활제 역할을 통하여 결정이 각형의 특정 방향으로 뾰족하게 성장하는 것을 억제하고 타원 형태로 성장하도록 유도하는 효과가 있다. 또한 티타늄 표면 전체에 고르게 결정들이 형성되는 여부를 확인하기 위하여 JGB 첨가제를 첨가하지 않은 군과 분극효과가 높았던 30 ppm 첨가군을 시편의 끝단에서 1 mm 떨어진 구간과 중앙부를 비교한 결과를 그림 4에 나타내었다.
JGB 무첨가군에서는 중앙부 대비 끝단 부분에 결정의 생성이 상대적으로 많음을 확인할 수 있으며, 30 ppm 첨가한 군에서는 무첨가군 대비 결정이 표면 전체에 균일하게 분포를 하고 있는 양상을 보이고 있다. 이는 그림 1에서 확인 하였듯이 도금 초기부터 평활제가 도금 표면 전면에 핵생성을 유발하고 균일한 성장을 위한 역할을 하고 있음을 확인 할 수 있다. 이러한 첨가제의 역할은 도금이 진행되는 동안 지속적으로 이루어지고 이로 인하여 전착층의 전기적, 기계적 특성에도 영향을 미칠 것으로 생각된다.
그림 5는 12 µm 두께로 전착한 시료를 30도 회전하여 표면 형상을 분석한 결과이다. JGB 첨가제를 첨가하지 않은 군의 표면에는 도금이 매끄럽게 균일하게 이루어지지 않았다. 특히 일부 표면에서는 함몰 및 핀홀이 관찰되었으며, 이는 물리적 특성에 악영향을 미칠 것 생각된다. JGB 첨가제를 10 ~ 30 ppm 첨가한 군에서는 균일한 전착층이 형성되었으나, 첨가량이 40 ppm 이상 조건에서는 균일한 표면 위로 미세한 결정들이 돌출되어 생성되는 것을 확인할 수 있다. 이러한 현상은 60 ppm 첨가한 표면에서 확연하게 나타나고 있으며 일부 표면에서는 1 µm 이상의 돌기가 표면에 돌출되어 있다. 이는 첨가량이 일정수준 이상인 경우에는 오히려 첨가제간의 상호작용에 악영향을 미치며, 균일한 전착층 형성에 저해요인으로 작용됨을 알 수 있었다.
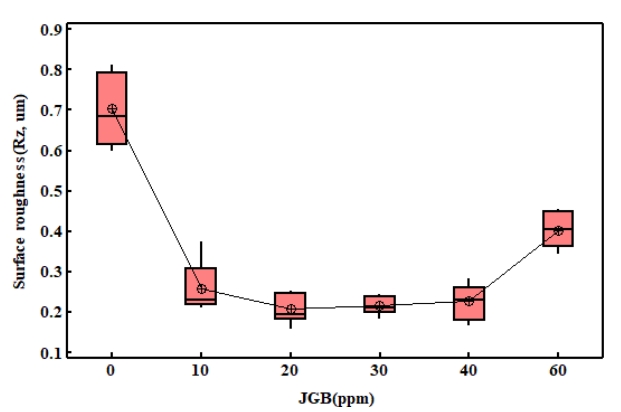
그림 6은 12 µm 전착층 표면의 거칠기를 측정한 결과이다. 무첨가군의 표면조도가 높게 측정되었으며, 이는 불균일한 표면 및 함몰, 핀홀 등의 영향으로 여겨진다. JGB 첨가량이 10 ~ 40 ppm의 경우에는 무첨가 대비 평균 68 %로 감소한 결과를 보이고 있으며, 이들 그룹들 사이에는 p-value 값이 0.134로 표면거칠기 상의 유의차가 없다. JGB 첨가제를 60 ppm 첨가한 경우에는 표면에 돌기 등의 발생으로 측정장비의 오류가 발생하여 이상구간을 제외하고 측정한 결과로 실제 표면거칠기는 측정결과 보다 높은 거칠기를 가지고 있다.
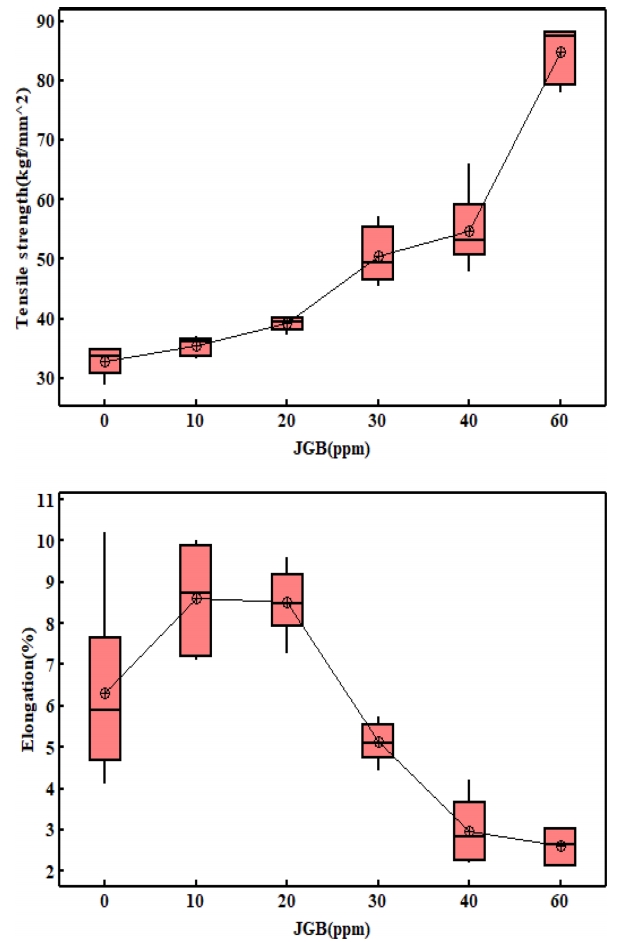
그림 7은 인장강도와 연신율을 측정한 결과이다. 평활제를 60 ppm 첨가한 경우 무첨가군 대비 인장강도가 157% 증가하였고 연신율은 59% 감소하였다. 인장강도의 경우 첨가량 증가에 따라 지속적으로 증가한 반면 연신율은 무첨가군 대비 10 ~ 20 ppm JGB를 첨가한 경우에 증가양상을 보인 후 감소하였다. 또한 무첨가군의 경우의 연신율은 약 4 ~ 10%로 넓은 분포를 보이고 있다. 이는 그림 5에서보면 표면에 함몰 및 핀홀이 존재하여 균일한 전착층이 형성되지 않아서라 생각 할 수 있다.
기계적 특성 및 전기적 특성과 결정구조와의 상관관계를 파악하기 위하여 XRD 분석 결과를 그림 8에 나타내었다. 무첨가 군에서는 (111) 픽이 상대적으로 높게 나타나고 있으며, JGB 첨가량이 증가하면서 (111) 픽의 감소와 (220) 픽의 증가 현상이 두드러지게 나타나고 있다. 첨가제에 따른 집합조직의 분율 및 변화를 확인하기 위하여 XRD 2θ scan 결과를 토대로 식 1을 통하여 계산한 Texture Coefficient (TC) 결과를 그림 9에 나타내었다.
여기서 I(hkl)과 I0(hkl)은 각각 실험시편과 표준분말시편의 (hkl)회절의 적분강도이며, n은 회절면의 총 수이고, Σ는 합을 의미한다. 만약, 모든 회절면의 TC가 1이면 결정 방위분포는 무질서하며, 임의의 (hkl)면의 TC가 1보다 크면 결정립의 (hkl)면이 기판면에 평행하게 되어 있는 우선 방위 (또는 집합조직)를 가진다. 본 실험에서는 (111), (200), (220), (311) 4종류의 회절면에 대하여 측정하였기 때문에 만일 (111) 회절면만 관찰되었다면 (111)의 TC는 4가 되고 나머지 회절면들의 TC는 0이 된다. 이 경우 전착층 집합조직의 전부를 (111)로 볼 수 있다 [24,25]. JGB 첨가제를 첨가하지 않은 경우에는 (200) 집합조직의 영역이 커서 TC값이 1.55(38.9%)로 가장 크며, (111) > (220) > (311) 순서로 나타났으며, TC값은 각각 0.92(23.0%), 0.90(22.4%), 0.63(15.7%)로 나타났다. 괄호안의 백분률 값은 전체를 100% 환산하여 해당집합조직의 분율을 나타낸 것이다. 그러나 JGB 첨가제를 첨가한 경우에는 (200) 집합조직이 10 ppm에서 일부 증가하였으나 이후의 첨가량에서는 급격하게 감소하는 양상을 보이고 있다. 반면 (220) 집합조직은 무첨가군에서 TC값이 1이하의 값을 보였으나, JGB 첨가량이 증가 할수록 증가하는 양상을 보이고 있다. Gittis [26]등에 따르면 구리의 각 면간의 표면에너지는 (111)면이 가장 작으며, (200), (220) 순으로 증가된다 하였다. 또한 (220) 집합조직으로 성장하는 경우 즉, 음극 표면에서의 원자들의 이동이 원활하지 않는 조건에서 높은 표면에너지를 가지는 면으로 집합조직이 우선 배향 된다고 하였다. 일반적인 평활제의 거동을 살펴보면, 음극표면에서 표면에너지가 높은 곳에서 첨가제가 흡착되게 된다. 이러한 영역으로는 edge, nodule 그리고 needle를 들 수 있다. 극성을 띄고 있는 평활제는 이곳에서 isolating layer를 형성하여 표면에너지를 감소시킨다. 따라서 needle이 성장하는 것을 억제하게 된다[27]. 그러나 JGB 첨가제를 일정량 첨가하게 되면 표면이 균일하여 음극표면의 표면에너지가 균일하게 되어 원자들의 이동이 원활하지 않게 되고 음극 표면 주위의 구리이온의 농도가 높아져 표면에너지가 높은 (220) 집합조직으로 결정들이 우선적으로 성장하게 되는 것으로 생각된다. 또한 Kim [1]등에 따르면 전착층내에 저조밀 집합조직인 (220)면과 (311)면으로 성장방향성이 증가하면서 인장강도가 증가한다고 하였다. 이러한 결과는 이번 연구에서 평활제인 JGB를 첨가함에 따른 인장강도 증가 현상과 일치한 결과를 나타내고 있다.
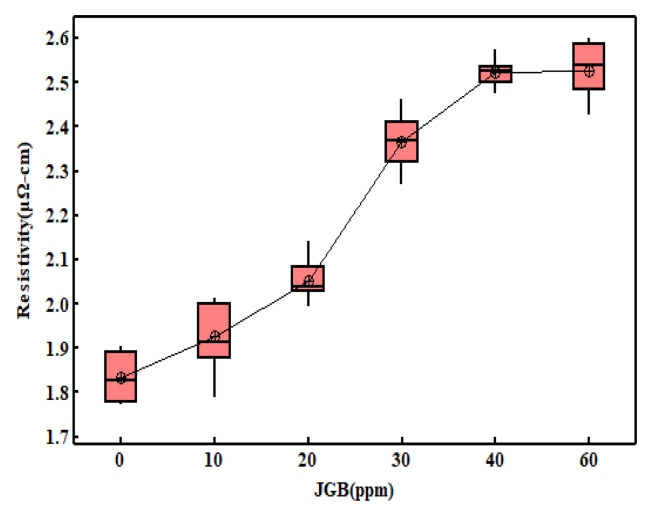
그림 10은 구리도금막의 중요한 물성으로 전기적 평가를 통해 전기비저항을 측정한 결과이다. 평활제를 60 ppm 첨가한 경우에 무첨가 군 대비 37.7%의 비저항 값이 증가하였다. kuan [28] 등에 따르면 표면 거칠기를 활용하여 비저항 값을 산출 할 수 있다고 하였으며, 비저항은 표면 거 칠기에 비례하다고 하였다. 그러나 이번 실험에서는 무첨가군 대비 JGB를 첨가한 경우 표면거칠기가 감소하였기에 표면거칠기의 영향은 아닌 것으로 생각된다. 또한 평활제는 억제제와 마찬가지로 도금을 억제하는 역할을 하며, 억제 역할을 하기 위하여 도금 표면에 흡착하게 된다. 평활제는 이러한 흡착능력이 억제제, 촉진제보다 강하다고 하였다[14,29-32]. 다른 측면에서 이러한 강한 흡착력은 도금 내에 매몰(trap)이 쉽게 일어날 수 있을 것으로 판단되며, 이로 인해 도금층의 불순물의 농도가 높아질 수 있을 것으로 판단된다. Park 등[14]의 연구에 따르면 JGB를 사용하여 농도에 따른 구리 도금박막을 제작하여 각각의 JGB 조성에서 불순물의 농도를 분석 시 JGB 첨가제의 농도가 높아질수록 도금층의 불순물의 농도가 선형적으로 증가하고 이로 인해 도금박막 형성 후 결정립 성장이 억제된다 하였다. 본 연구에서도 JGB 첨가량이 0 ~ 40 ppm까지 증가하는 동안에는 선형회귀 근사식에서 R2 값이 91.4 %로 JGB 농도에 비례하여 비저항이 증가하는 것을 알 수 있으며, 40 ~ 60 ppm 구간에서는 유의차가 없었다. 도금과정에서 탈락되지 못하고 전착층 내부에 존재하는 불순물은 Matthiessen’s rule을 따라 불순물에 의한 비탄성 산란 충돌빈도의 증가로 설명될 수 있으며, 각각의 원자들이 구리 격자 내에 치환형 혹은 침입형으로 위치하여 전자의 비탄성 산란에 의해 전기저항이 증가하였다고 판단된다. 또 다른 요인으로는 JGB 첨가량이 증가할수록 (220) 집합조직 TC의 증가 현상과 비저항의 증가양상이 비슷한 경향을 보이고 있어 비저항의 증가 원인으로 집합조직의 변화 또한 중요한 변수가 될 것이라 생각된다. 집합조직의 변화로 결정립 크기의 변화가 이루어지며, 이러한 결정립의 크기 또한 비저항의 변화에 중요한 요인이 된다.
여기서 λ는 XRD 측정 시 사용한 구리 타겟의 파장으로 15.4056 nm이며, θ는 회절피크의 위치의 2θ의 절반 값이다. B는 시편에서 각각 측정한 반가폭을 이용하여 구하였다.
JGB 평활제 첨가로 전착층 표면에서 결정들이 성장하는 것을 억제하여 결정립 크기를 감소시켰고 (311), (220) 집합조직에서의 감소 현상이 두드러지게 나타나고 있으며 이렇게 감소한 결정립의 크기가 나노사이즈임을 알 수 있다. Huh 등[24]에 따르면 나노사이즈의 결정립에서는 경계면의 비탄성산란에 의해 비저항이 증가하게 한다하였다. 결정립 크기의 감소는 결정립계면 분율의 증가로 비탄성산란이 증가하여 비저항도 비례하여 증가하게 된 것으로 보인다. 또한 이러한 결정립의 크기 감소로 구리 박막의 표면 경도의 증가는 Hall-Petch 효과로 설명할 수 있을 것이다. Hall-Petch 효과에 의하면 마이크로미터 수준에서 결정립의 크기가 작아질수록 재료의 기계적 특성이 향상된다[35,36]. 결정립의 미세화로 인하여 Hall-petch 효과가 크게 작용하여 인장강도가 급격하게 증가하였다.
4. 결 론
황산구리도금액을 이용한 고전류밀도 전해도금 공정에서 평활제 JGB 첨가제하여 초기핵생성 관찰 및 전착층의 표면특성, 기계적 특성 및 전기적 특성을 분석을 통하여 다음과 같은 결론을 얻었다.
1. 평활제 JGB 첨가제를 첨가한 경우 초기 핵생성과정에서 전착층의 가장자리와 중앙부에서 고르게 결정이 형성되어 성장하는 효과가 있다. 이러한 효과는 12 µm 도금층 표면에 무첨가군에서 발생되는 함몰, 핀홀 감소효과로 균일한 전착층을 얻을 수 있으며 JGB 첨가량이 10 ~ 40 ppm의 경우에는 무첨가 대비 평균 68%로 감소하였다.
2. JGB 첨가제는 초기 핵생성과정에서 결정성장을 억제하고 핵생성을 촉진하여 표면에너지가 높은 (220) 방위로의 결정성을 촉진하고, 결정립 크기의 감소로 인하여 무첨가군 대비 인장강도는 최대 157% 증가하였으며, 연신율은 59% 감소하였다.
3. 구리 전착층의 중요한 물성인 전기비저항은 결정립 크기의 감소, 집합조직의 변화 및 첨가제의 내부 혼입 등으로 인하여 증가하였다. 따라서 첨가량을 적절히 조절하는 것이 필요하다.
결과적으로 고전류밀도 전해도금에 JGB를 평탄제로 첨가하기 위해서는 20 ~ 30 ppm 범위에서 첨가하여 표면의 함몰을 감소시키고 평활한 전착층을 제조하는 것이 타당하다.