1. INTRODUCTION
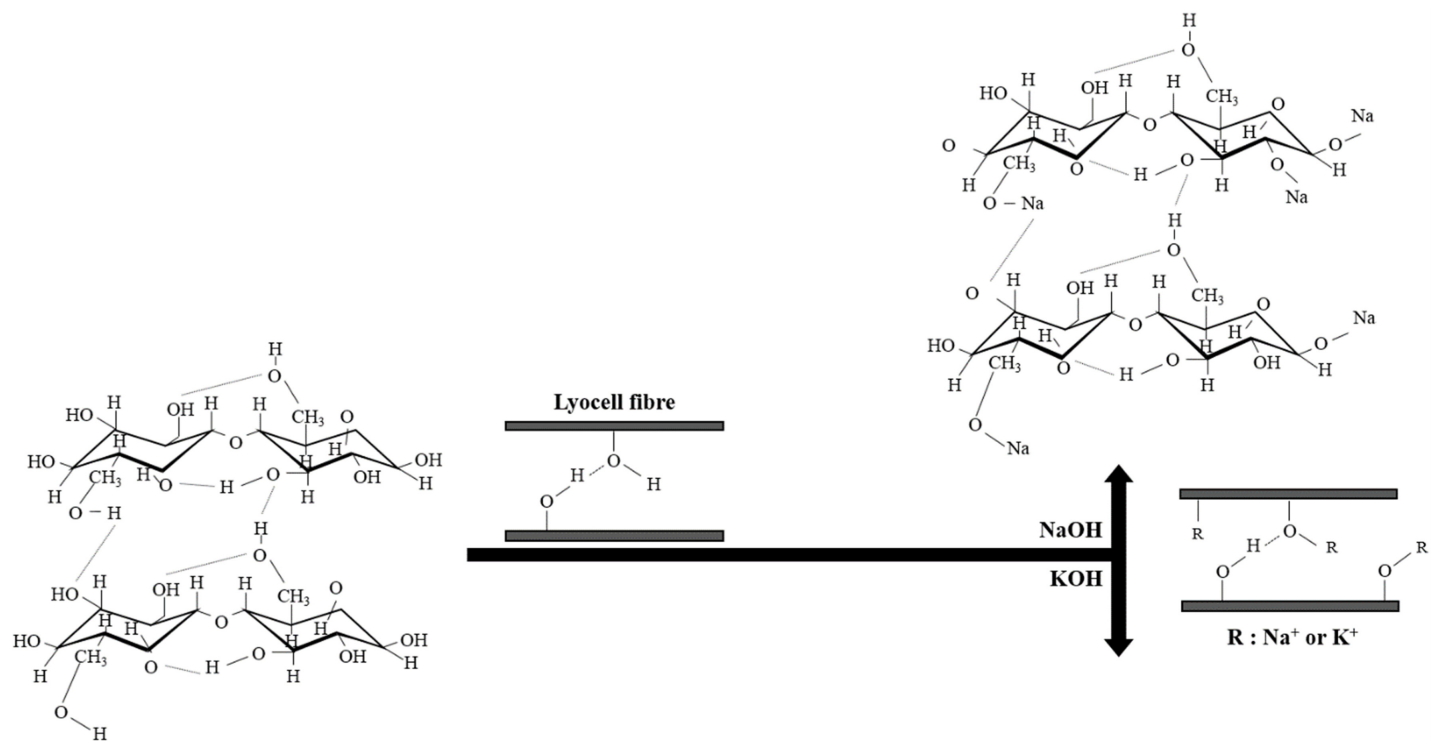
Lyocell is a regenerated cellulosic biopolymer fundamental to the development of membranes, films and fibres [1-3]. The fibre form comes from the wood pulp of eucalyptus dissolved in N-methyl-morpholine-N-oxide, which adheres to the cellulose microfibrils [4]. Lyocell has long attracted attention, particularly for its high degree of nanoscale modification. Alkali alters the physiochemical behaviour of lyocell, viz., the dimensional, mechanical, and supramolecular properties of cellulose II fibres [5-8]. Different pretreatment approaches have been introduced to alter the structure of biomass using NaOH and KOH (Figure 1). Some earlier studies employing optical microscopy and X-ray diffraction (XRD) have provided insights in terms of crosslinking between the alkalis and fabric forms of lyocell, and for characterizing the subset of physiochemical dynamics. Currently, lyocell approaches are being integrated with precise engineering, with the result that they possess a Brunauer-Emmett-Teller (BET) surface area of more than 1,000 m2 g-1. However, previous results based on samples with less than 1,000 m2 g-1 may hinder their adoption in applications. Accordingly, a distinct need exists to determine how alkalis affect the BET surface areas of samples.
One of the drivers of nanocellulose technology is lyocell-based activated carbon fibres. We designate these ACFs for convenience. ACFs are produced in activated fibrous form from fibres and are ideally suited for microelectrodes, nanosheets, and as adsorbents due to their outstanding chemical stability, high flexibility, and small thermal expansion coefficient [9-13]. A theoretical and experimental cornerstone, to determine the supramolecular shift of recently reported ACFs, is critical to understanding how crystallinity varies with different alkali dosages. In a proof-of-principle experiment, we demonstrate the degree of crystallinity of ACFs via NaOH and KOH based fibre modification. We take advantage of the X-ray diffraction (XRD) technique to analyze the difference in structural composition produced by various fibre modifications. A thermo-gravimetric analysis (TGA) was also employed to cross-validate and assess the degree of crystallinity. The paper is organized as follows. In Sec. II, we introduce the method of sample preparation and procedures. After describing the experimental setup and techniques used to measure the full probability distributions of the output ACFs, Sec. III is devoted to the analysis of various modifications. Conclusions and further perspectives are given in Sec. IV. The as-prepared ACFs have hierarchically interconnected macro-, meso-, and micropores with a decent surface area. Taking the XRD and TGA results together, we are confident that ACFs de facto obey the general characteristics of lyocell-based fibres, regardless of their BET surface area. Additionally, we also suggest the optimum proportion of impregnating elements under the concept of precise engineering.
2. EXPERIMENTAL
2.1 Synthesis and structural characterization of samples
A 100% lyocell fibre was kindly supplied by Hyosung Corporation (South Korea) and used in this work. The denier and filament were 1650d and 900f grade, respectively. The NaOH and KOH alkalis were all analytical reagents and the solutions were all formulated in deionized water. The alkali treatments were performed on fabric pieces of a given dimension (3 cm × 3 cm) using a batch method. The pieces were padded through the alkali solutions with a nip pressure of 1 bar. The first alkalis treatments employed 10~25 % of NaOH and KOH for 3 h at ambient room temperature, respectively. The designated proportion of alkalis is shown in Table 1. ACF-W is a counterpart reference sample treated with deionized water. We limited our dosage to 25 % to prevent the possible reduction of crystallinity. The secondary treatment was conducting using 4 % each of KOH and H3PO4 for another 3 h [14]. Note that KOH was applied to design a porous surface on the sample, while H3PO4 was applied for better yield in the high temperature range during carbonisation and oxidation [15-16]. Afterwards, the samples were neutralized in deionized water for 10 trials and linedried overnight. An activated form of the samples was prepared using a three step procedure which included oxidation (stabilisation), carbonisation, and activation.
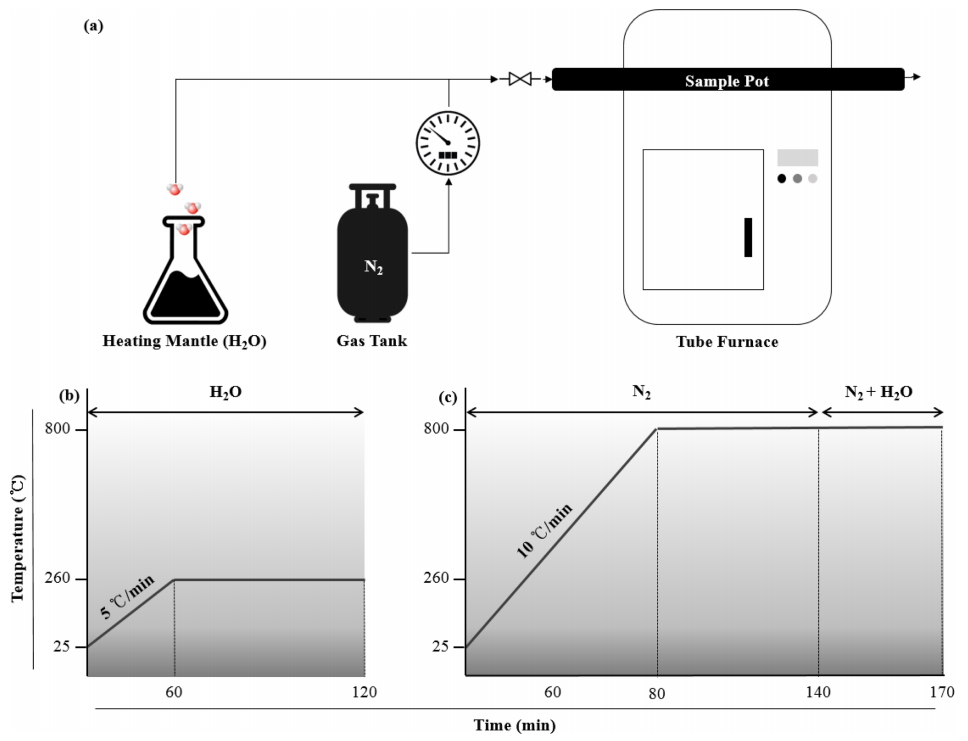
The schematic diagram in Figure 2(a) shows the overall process. N2 gas is released from the N2 gas tank for carbonization, and H2O from the heating mantle was included once the activation started. The first step was to oxidize the samples at a constant temperature of 300 °C with a heating rate of 5 °C min-1 for 1 h (Figure 2(b)). Subsequently, the samples were heated at a constant rate of 10 °C min-1 in the presence of a N2 stream (0.5 L min-1) up to 800 °C for 1 h (Figure 2(c)). Finally, the samples were activated for 30 min at the same temperature via a mixture of H2O + N2 flow. The BET method (N2-BET) revealed that all the modified samples reached more than 1,000 m2 g-1 (Micrometrics, ASAP2460) [17]. These numerical values suggest that a higher dosage of alkali solutions of either NaOH or KOH can result in a higher BET surface area compared to ACF-W. In all circumstances, a treatment of NaOH revealed higher BET surface characteristics than KOH. As depicted in Figure 3, the SEM images also supported that the 25 % NaOH treatment produced the most porous surface structure among all variations.
2.2 Measurements
The crystalline structure of the samples was determined using XRD (Bruker, D8 Advance) with monochromatic CuKa radiation (λ = 0.1542 nm) in the Bregg-Brentano reflection geometry [18,19]. The analysis was conducted via a designated condition with a step size of (0.02°), 2θ range (which varied with the impregnating material), and step time (0.1 sec). The degree of crystallinity (DC) was calculated as the ratio between the corresponding area to the crystalline phase and the total area on the curve, written as Eq. 1;
where IC is the crystalline phase and IT is the total area under the XRD pattern. The thermal stability of the samples was measured through TGA (Sinco, N-1000). The samples were scanned from ambient room temperature to 300 °C using N2 gas with a heating rate of 5 °C min-1 for 100 min.
3. RESULTS AND DISCUSSION
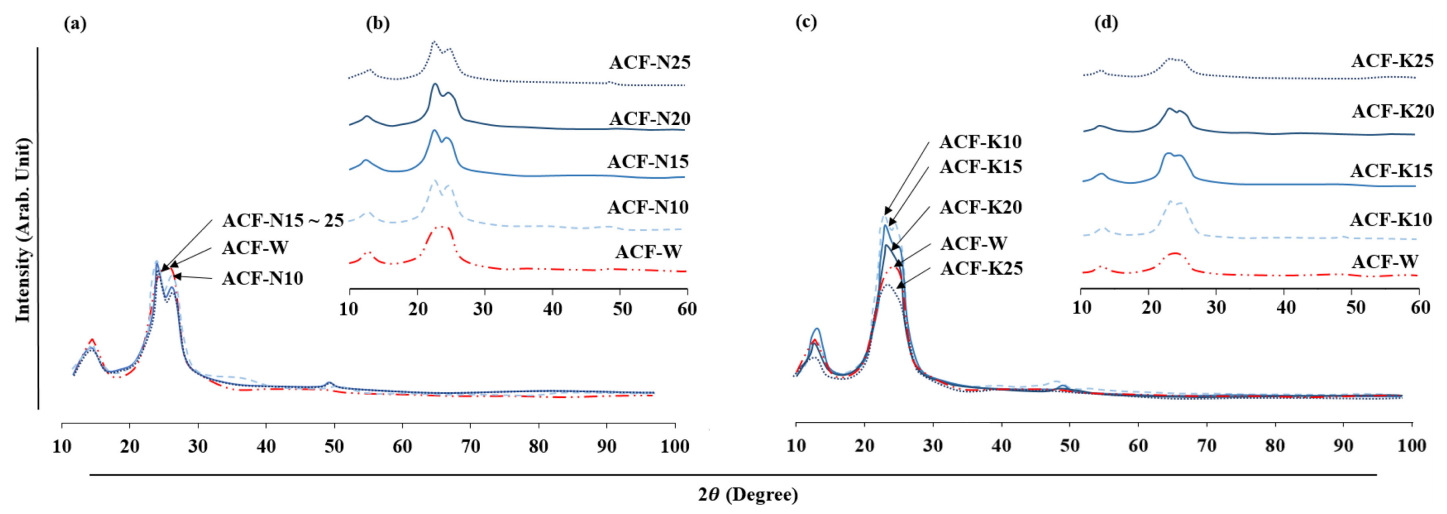
Figure 4 provides the XRD spectra of samples, with the peaks labelled to indicate their crystal lattice assignments. The spectra of the modified (ACF-Nn and ACF-Kn) samples are clearly different than the pristine sample (ACF-W). The peaks for the shoulder (2θ = 20°) and the top (2θ = 22.5°) in the intensity profiles of the alkali-treated samples revealed a tendency to become sharper than that of ACF-W. Interestingly, the intensity increased with an increase in KOH content of 20 % or less, compared to ACF-W, but the opposite result occurred in ACF-K25. Further, a general trend of anti-correlation between the KOH dosage and intensity was observed. Here, the ACF-K10 showed the highest DC increment with 2.1 % better crystallinity than ACF-W (Table 2). Meanwhile, the resulting intensity after NaOH treatment was found to decrease in all cases compared to ACF-W. Such decrements are mainly due to the alkali treatment. The alkali modification swells the lyocell fibres and weakens the dense structure and intermolecular bonds of the fibrous polymer chain [18]. This results in a rearrangement between the cellulose molecules, and drives a transformation from an antiparallel to a parallel conformation.
A characteristic band of 2θ = 12.3° was observed in ACF-W, which corresponds to the (1 0 1) lattice plane. However, there was only a single broadband between 2θ = 20° ~ 22°, indicating that a premature to classify ACF-W as a cellulose II crystallite. In the modified samples, the characteristic peaks of cellulose II were observed Figures 4(b) and 4(d). The observation of major peaks with diffraction angles assigned to the (1 0 1) and (0 0 2) lattice planes suggest that ACF-Nn and ACF-Kn yield fair characteristics of the cellulose II crystallites, which is consistent with previous reports [19]. Overall, ACF-Nn exhibited more preferable cellulose II crystallites than ACF-Kn, since Na+ has a greater hydration radius compared to K+ ions [20-22]. It appears that NaOH treatment may reduce the DC while such change in structure may alter crystallites into a more distinct form of cellulose II. In sum, both NaOH and KOH had a visible influence on the DC shift, and thus were vital to controlling alkali concentrations to achieve the target crystallinity for further use.
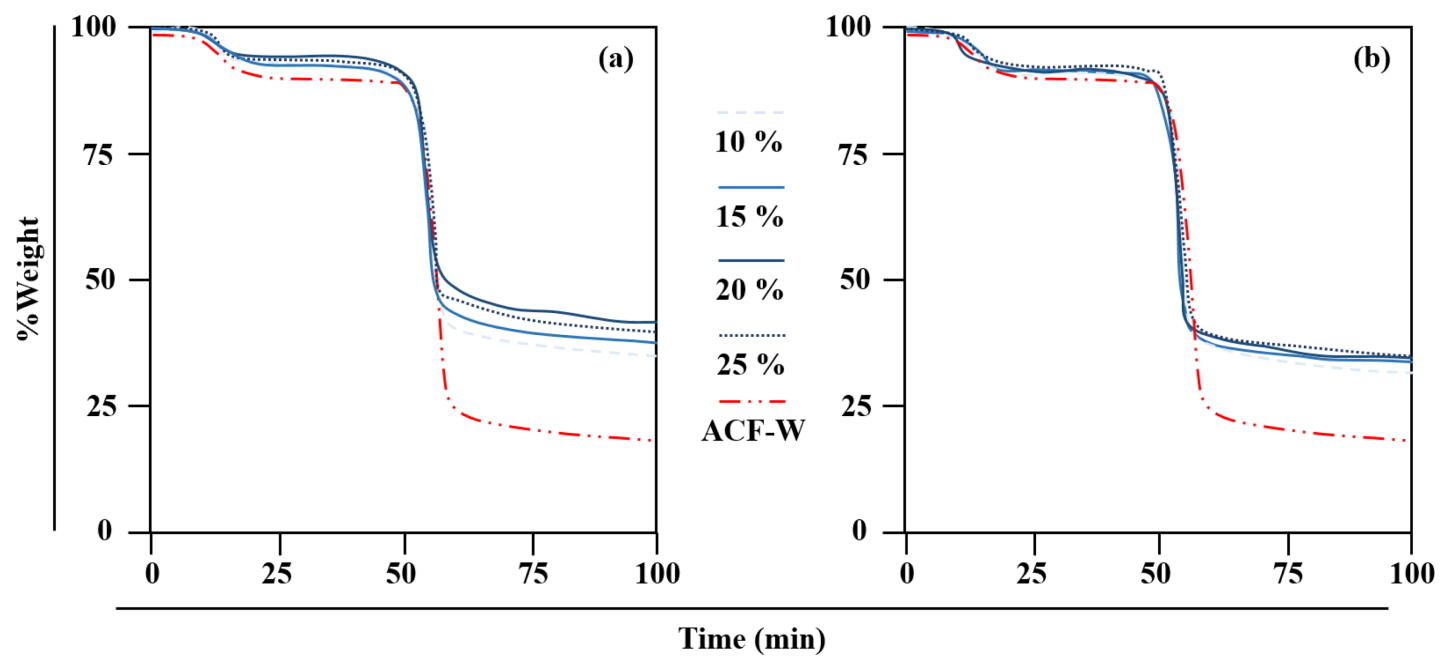
After treatment in alkalis, the samples were assessed by TGA to further investigate their conformation resulting from thermal gravimetric mass loss. In general, lyocell-based fibres show two phases of weight loss, due to dehydration and decomposition of the cellulose [23]. Figures 5(a) and 5(b) show that the samples obeyed general properties, with a major weight loss at temperatures between 150 °C and 200 °C after dehydration. Comparing the alkali treatments performed in this study, both NaOH and KOH demonstrated a strong upward linear relationship compared to ACF-W. It is therefore suggested that the thermal stability of the treated samples was improved via the fibre-reinforcing effect [24]. Under similar treatment dosages, ACF-Nn holds better thermal stability than ACF-Kn. It was also found that 15 % and 20 % dosages resulted in the best thermal stability in ACF-Nn and ACF-Kn, respectively.
4. CONCLUDING REMARKS
In conclusion, we studied the effect of alkali treatment of ACFs with high BET surface areas. By probing the domain orientations of samples using XRD and TGA, we were able to map the findings as follows.
(1) A 25 % dosage of NaOH provided the highest BET surface area with the most porous surface structure compared to other samples.
(2) ACF-K15 showed the highest DC, while the use of 15 ~ 25 % NaOH resulted in the most distinct form of cellulose II.
(3) The flame retardancy of samples was improved significantly after alkali treatment. A 15 % of NaOH was found to provide the best thermal stability among all samples.
(4) Combining all the presented strategies, these findings offer a fundamental route to control the behaviour of ACFs that may aid in the growing field of highly porous material research using lyocell-based fibres.