1. INTRODUCTION
In recent years, environmental pollution and its impact on human health have attracted the special attention of all countries in the world, especially to address the pollution of ocean water and land surface by oil spills. Every year, the world experiences a series of oil tanker accidents which result in millions of tons of oil being spilled into the sea, and serious consequences for marine resources and the environment. A number of effective and highly feasible solutions have been proposed by scientists to deal with oil spills. The most commonly used oil spill remedies today involve the use of oil booms, skimmers, and dispersants [1]. Natural recovery, chemical stabilization of oil, and bioremediation have been also investigated to solve oil pollution [2].
Among various approaches used for oil spills, the most common measure is the use of adsorbents, because they are most effective for small spills or for managing the remnants of a larger spill [3,4]. Inorganic mineral materials and natural/synthetic organic materials are the most widely used adsorbents [5]. Among them, synthetic polymer fibers are particularly suitable because of their controllable hydrophobicity, lightness, low cost, and simplicity of manufacture [6]. In particular, porous fibers which have a large specific surface area have increased efficiency and ability to absorb oil [7,8]. Thus, novel adsorbent materials with suitable hydrophobicity are needed for highly efficient oil uptake both at the surface and under water.
The development of oil adsorbents composed of a suitable material like silica is being pursued as promising research. Silica is the most abundant component in the earth’s crust, and silica is considered a safe and non-toxic element for preparing nanostructured materials for useful applications. To fabricate organic and inorganic nanofibers, electrospinning (ES) is a simple, fast, and versatile process [9,10]. Though porous silica particles with elastomeric coating layer or aerogels have previously been synthesized for oil adsorption, a vapor-phase treatment or supercritical processes are required to prepare such functional materials for successful applications [11,12].
Fibrous materials with porous micro-structures can also be employed as efficient adsorbents. The void spaces between the fibers as well as macropores can provide hierarchically porous structures [13]. There are previous studies on the synthesis of such fibrous materials by centrifugal spinning, but silica fibers can be also prepared by more economical apparatus using electrospinning systems [14,15]. Additionally, the use of PS nanospheres with different diameters makes it simple to adjust the desired hole size in the fibers. Nonetheless, it is still challenging to prepare macroporous silica fibers with hydrophobic properties as oil adsorbents, which require surface modification using the proper silane coupling agents.
In this study, we fabricated porous silica fibers by electrospinning for application as an oil adsorbent to remove contaminant oil. Using tetraethylorthosilicate (TEOS) as the starting material, the spinning solution was prepared by adding polystyrene (PS) nanospheres synthesized by emulsion polymerization to form macropores. After electrospinning under high voltage and subsequent heat treatment, surface modification was carried out to fabricate hydrophobic silica fibers for oil adsorption. The amount of oil adsorbed by the porous fibers was studied using silica fibers with spherical macropores of controllable size. Additionally, the effect of surface modification was studied using various silane coupling agents with different functional groups, such as methyltrichlorosilane (MTCS), phenyltrichlorosilane (PTCS), dodecyltrichlorosilane (DTCS), butyltrichlorosilane (BTCS), octyltriethoxy-silane (OTES), and (3-mercaptopropyl)trimethoxysilane (MPTMS). Performance was determined by measuring the water contact angle and the adsorbed amount of oil.
2. EXPERIMENTAL
2.1 Materials and Reagents
For the synthesis of the polystyrene nanospheres, polyvinylpyrrolidone (PVP K30, MW 40,000) was used as a stabilizer and purchased from Junsei Chemicals. Ethanol (99.9%, HPLC grade) was used as a reaction medium and bought from Daejung Chemicals. Styrene monomer (99%) and initiator like α , α '-azobis isobutyronitrile (AIBN, 99%) were procured from Samchun and Junsei Chemicals, respectively. [2-(Methacryloyloxy) ethyl] trimethyl-ammonium chloride solution (MTC, 75% aq) was used as a cationic monomer and obtained from Aldrich Chemicals.
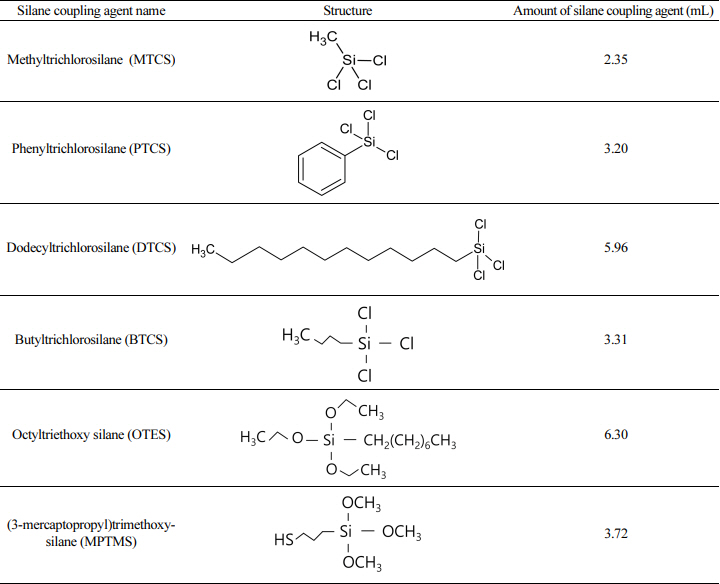
To prepare the spinning solution, tetraethylorthosilicate (TEOS, 98%) bought from Aldrich Chemicals was used as the starting material. For the gelation of the precursors, hydrochloric acid (0.1 N) was purchased from Daejung Chemicals, and polyvinylpyrrolidone (PVP360, MW = 360,000 g/mol) as an additive in the feed solution was purchased from Sigma Chemicals. For surface modification, methyltrichlorosilane (96%), phenyltrichlorosilane (97%), dodecyltrichlorosilane (95%), butyltrichlorosilane (99%), triethoxy(octyl)silane (97%), and (3-mercaptopropyl) trimethoxy-silane (95%) were purchased from Aldrich Chemicals. To test the oil adsorption capacity of the porous silica fibers, KF-96 silicone oil (100CS) was obtained from Shin-Etsu.
2.2 Synthesis of polystyrene nanospheres as templating materials
Monodisperse polystyrene nanospheres with diameters of 250, 430, 600, 870, and 1000 nm were synthesized by dispersion polymerization. After heating the batch reactor to 70 °C, a suitable amount of ethanol containing polyvinylpyrrolidone (PVP), styrene, and an aqueous solution of MTC were added to the reactor while stirring at 129 to 345 rpm, according to the composition summarized in Table 1. After nitrogen purging for 1.5 hours, AIBN was added to the reactor for polymerization for 20 hours. After the suspension was obtained, it was filtered and resuspended in ethanol with a solid concentration of 15 wt%.
2.3 Fabrication of fibers by self-assembly strategy during electro-spinning
The solution for electrospinning was prepared as follows. First, the polystyrene nanospheres in ethanol with a solid concentration of 15 wt% (7 ml) were sonicated for at least 30 minutes. Then, 1g of PVP was added and stirred for about 1 hour. Subsequently, 1.8 mL of TEOS was added to the PVP solutions and was stirred for 30 min. Then, a different amount of 0.67 mL of 0.1N hydrochloric acid was added, which was stirred for an additional 30 min. All these processes were stirred at 90 rpm at room temperature.
A syringe pump (EP100, NanoNC) was used to push the resulting spinning solution through the metal nozzle (0.5 mm ID, 0.8 mm OD, NanoNC) at a constant feed rate (15 μl/min). The fixed distance between the nozzle and the collector was 17 cm. A high voltage power source (NNC-HV60, NanoNC) was operated at 12 kV throughout the process. A flat-type collector (SUS sheet) was used to collect the resulting fibers. During the electrospinning process, the humidity was controlled at 20 to 25% at room temperature. To synthesize porous silica fiber, calcination was performed using a box furnace at 500 °C for 5 hours to remove the PS nanospheres.
2.4 Surface modification of porous silica fibers
During surface modification using silane coupling agents, 0.6 g of macroporous silica fiber was suspended in 50-mL toluene, followed by the addition of 0.02 mol of MTCS, PTCS, DTCS, BTCS, OTES, or MPTMS (Table 2, as determined on a 1x basis). Then, heating was carried out at 70 °C for 3 hours, while refluxing during stirring at about 200 rpm. After completion of the modification reaction, the solid samples were collected and dried at room temperature for at least one day before the adsorption of silicone oil.
2.5 Oil adsorption capacity of the porous silica fibers
To analyze the oil adsorption capacity of porous silica fibers it is possible to measure adsorption capacity by continually dropping organic solvents or oils until saturation point, and looking for a change in the solid-like property of the oil-silica mixture, to determine the adsorption equilibrium [16]. In this study, two types of oils were used to check the oil adsorption, based on the color change of the surface-treated fibers. Usually, a colorless silicone oil was used. Sudan III was mixed with oil to stain the oil a red color [17].
First, 1.0 g of the surface-modified silica fiber was placed in a glass beaker. Then, silicone oil or the crimson mixture of silicone oil and Sudan III were dropped slowly into the beaker at a slow rate of 0.01 g/s and stirred until the fibers were no longer able to absorb more oil. When this was visually observed, the oil adsorption process was stopped until the mixture began to turn into a slightly sticky state, like vaseline. Special care was taken to minimize the contact area between the adsorbent fibers and the cup during stirring. The amounts of adsorbed oil was compared after adjusting the size of the PS nanospheres, or by changing the amount of silane coupling agent like methyl trichlorosilane during surface modification.
2.6 Synthesis of meso-macroporous silica particles
In a typical synthesis, the 430 nm diameter PS nanospheres were centrifuged at 13,000 rpm for 30 minutes and redispersed in ethanol by sonication. The precursor solution containing TEOS was prepared by mixing a small amount of hydrochloric acid solution (0.01 N, 1.69 g) with PS nanospheres in ethanol (7.5 g, 30 wt%) for 30 minutes by stirring, followed by the addition of TEOS (3.255 g) under stirring at 200 rpm for 1 hour. Then, an aqueous solution of Pluronic F127 surfactants (4.2688 g, 10 wt%) was mixed with the precursor solution composed of TEOS, aqueous HCl solution, and PS nanospheres in ethanol for another 30 minutes to prepare the dispersed phase. The continuous phase was prepared by mixing the emulsifier (ABIL® EM 90) in tetradecane (3 wt%). Then, the dispersed phase was poured into the continuous phase at a volume ratio of 1:3 followed by homogenization for 60 seconds using a homogenizer (Wisetis®). Then, evaporation of the dispersed phase was carried out by heating at 90 °C for 60 minutes under mild stirring at 300 rpm. The resultant composite particles were collected by natural sedimentation, then washed with hexane three times to remove the emulsifier and remaining hexadecane.
2.7 Characterizations
The morphologies of the porous silica fibers were characterized by field emission scanning electron microscopy (CX_200 Plus, COXEM). SEM images were captured at acceleration voltages of 15 to 20 kV. Before imaging, the samples were coated with an Au layer for 180 seconds at 4 mA. FT-IR spectra of the fiber samples were measured using an FT-IR spectrometer (Nicolet iS5, Thermo Fisher Scientific co. Ltd).
3. RESULTS AND DISCUSSION
3.1 Electrospinning-assisted self-assembly of porous silica fibers
The structure and morphology of the nanofibers fabricated by electrospinning are determined by a set of parameters related to the solvent, polymer solution, processing parameters, and ambient conditions [18]. The two main factors for a successful spinning process are having a polymer with adequate molecular weight, like PVP, and an available solvent to dissolve the polymer. The solubility and volatility parameters of the solvent directly affect the formation of the homogeneous polymer solution [19,20]. Very low volatility is not suitable for spinning, because the fibers will still be wet when they are deposited on the collector. If the volatility is too high, the jet can solidify as soon as it exits from the spinneret.
In addition to the molecular weight of the polymer and the type of solvent in the spinning solution, the spinning ability of the polymer solution also depends on the concentration and conductivity of the feed solution. To successfully obtain fibers, a minimum concentration of polymer is required to initiate the transition from electrospray to electrospinning. If the concentration is below this limit, the interactions between the polymer chains will be too small and they will be unable to overcome Rayleigh instability, causing the jet to break-up into droplets, producing fine particles instead of continuous fibers. Conversely, no jet will be formed if the concentration is too high, due to the increased viscoelastic force [21,22].
In this study, silica fibers were fabricated by the sol-gel method using an electrospinning process [23]. A high voltage power was applied to the spinning solution to form fibers. Silica fibers containing PS nanospheres as templates were converted into macroporous silica fibers after calcination. The pore size could be controlled between 250 to 1000 nm by adjusting the diameter of the PS nanospheres in the spinning solution.
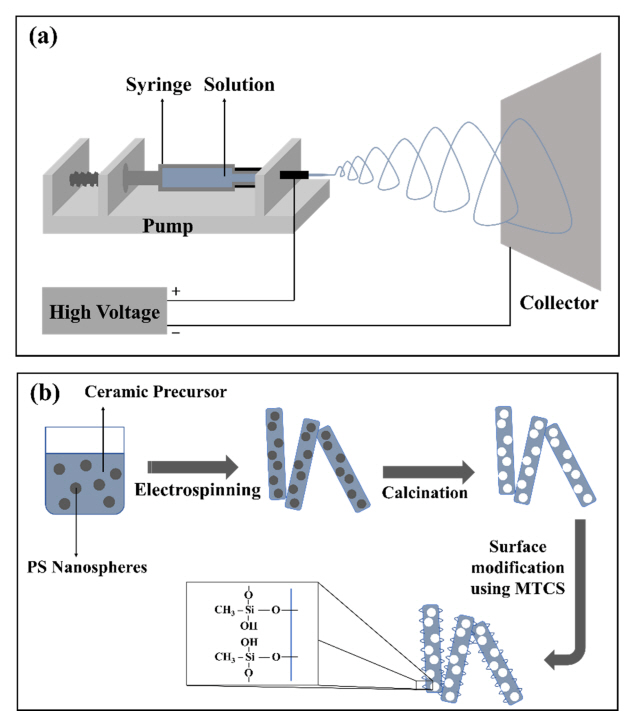
Electrospinning consists of an electrodynamic process in which a drop of liquid is electrified to create a jet, followed by stretching and elongation to produce fibers, as illustrated in Fig 1(a) [24,25]. Before the resulting self-assembled fibers become attached to the flat-type collector, the volatile medium is completely evaporated by the atmospheric environment.
The formation process of porous silica fibers is presented schematically in Fig 1(b). Composite fibers of PS nanospheres and silica could be formed during electrospinning by the self-organization of the silica precursors, polymer, and templates. To prepare the spinning solution, tetraethylorthosilicate (TEOS), polymer, and PS nanospheres were dispersed and mixed in a volatile organic solvent (ethanol), followed by the addition of small amount of hydrochloric acid for gelation.
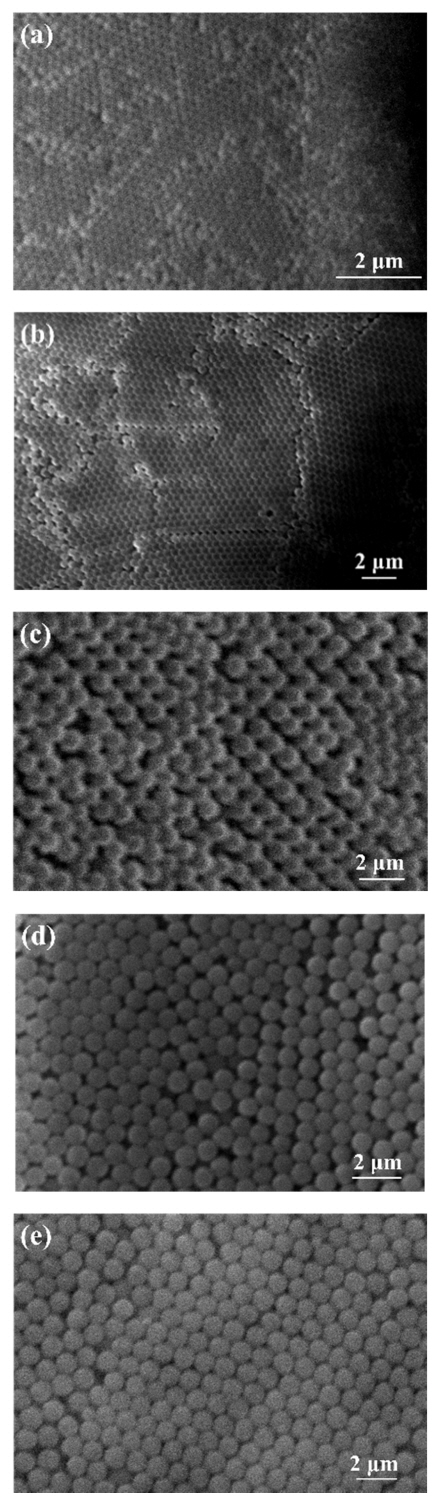
Fig 2 shows the morphologies of the PS nanospheres, synthesized by dispersion polymerization with different diameters. Here, the PS nanospheres were synthesized using ethanol as a reaction medium. To form the desired size, the synthesis conditions were adjusted by changing the amount of reagent involved, such as PVP, styrene, or MTC, or the environmental conditions such as temperature and agitation rate. To increase the size of the PS nanospheres, the amount of PVP stabilizer was reduced, as summarized in the reaction conditions #2 in Table 1. In this case, 430 nm diameter was achieved. A further increase in PS nanosphere diameters was accomplished by increasing the amount of styrene, and 600-nm PS nanospheres could be synthesized from the reaction conditions of #3 in Table 1. The particle diameter could be increased again by further reducing the amount of PVP stabilizer, achieving 870-nm PS nanospheres from the reaction conditions of #4 in Table 1. Finally, to synthesize PS nanospheres of 1000-nm size, the polymerization was carried out with condition #4, with a slight change by reducing the amount of PVP to 2.188g (condition #5 in Table 1).
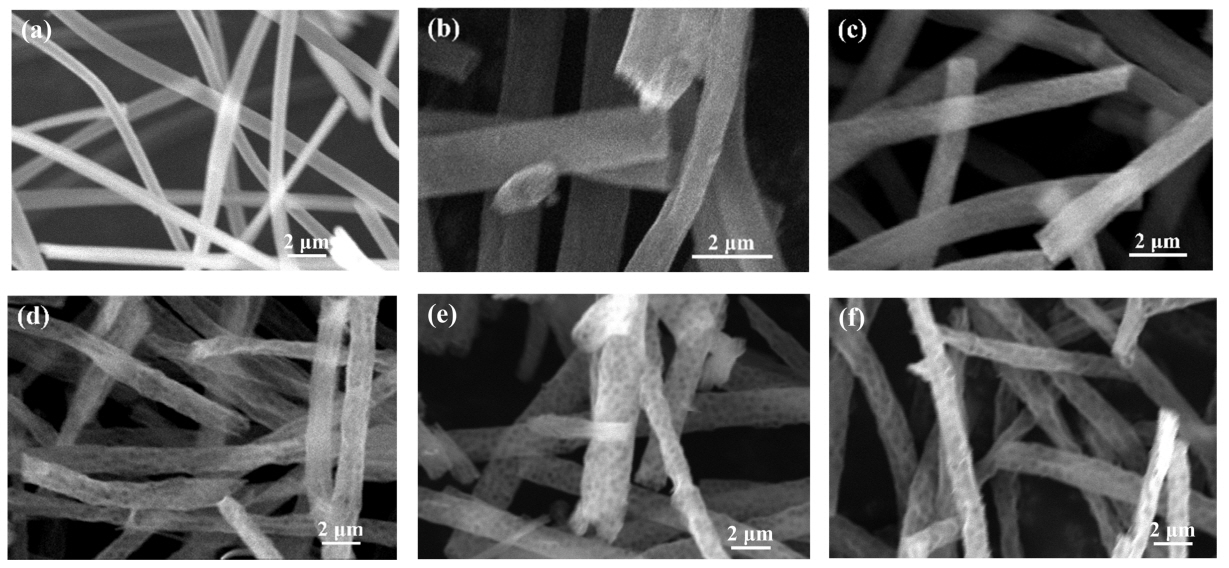
Fig 3(a) shows an SEM image of the nonporous silica fibers after calcination. In Fig 3(a), the morphology of the silica fibers in the absence of PS nanospheres is shown, and the existence of macropores cannot be confirmed. Fig 3(b), 3(c), 3(d), 3(e), and 3(f) present SEM images of macroporous silica fibers where the pore size was controlled using PS nanospheres with diameters of 250, 430, 600, 870, and 1000 nm, respectively. The PS nanospheres were removed during the heat treatment, leaving a number of air holes forming porous silica fibers, increasing the porosity of the fibers. The macroporous silica fibers in this study exhibited a large specific surface area. For instance, the BET surface area of the porous fibers fabricated using 430 nm diameter PS nanospheres was measured to be 741.0636 m²/g. This means that oil contaminants can be removed using a small amount of the fibers, due to their large surface area.
3.2 Fabrication of water-repellent surfaces using macroporous silica fibers
Because silica has a hydrophilic surface due to hydroxyl groups, it is not suitable for adsorbing oil. Thus, a silane coupling agent was used to modify the surface to give it a hydrophobic property. After surface modification with MTCS, the resulting silica fibers exhibited hydrophobic behavior because of the introduced methyl groups. In this condition, the porous fibers can be used as an oil adsorbent.
The structure of the silane coupling agent is generally expressed by the structural formula Y-Si-X3 [26]. The silane coupling agent has at least two different functional groups with different reactivity in the molecule. X represents a hydrolyzable group, which differs depending on the kind of coupling agent. For example, the X is Cl in the surface modification process using MTCS in Fig 1(b), and it has hydrolysis properties, which means it is hydrolyzed to the OH group.
Y is a reacting group that chemically bonds with inorganic materials such as glass, metal, and others. For instance, a vinyl group, an epoxy group, an amine group, and a methacrylic acid group can chemically bind to the organic material of various synthetic resins [27,28]. Thus, the silane coupling agent acts as an interfacial binder because it has a reactive group capable of bonding with a surrounding matrix due to Y, which represents an organic functional group. Because hydroxyl groups (-OH) exist on the surface of macroporous silica fibers, an alcoholysis reaction between the hydroxyl groups and X in the coupling agent results in covalent binding of the coupling agent, which transforms the surface hydroxyl groups to the other functional group, Y. This makes surface modification of the porous fiber possible, depending on the chemical nature of the Y group. Silane coupling agents on the treated surface reduce the amount of hydrophilic hydroxyl groups produced by the surface modification. Thus, the surface property can be changed from hydrophilic to hydrophobic, depending on the chemical property of the Y group in the coupling agent, to achieve a high oil adsorption capacity [29].
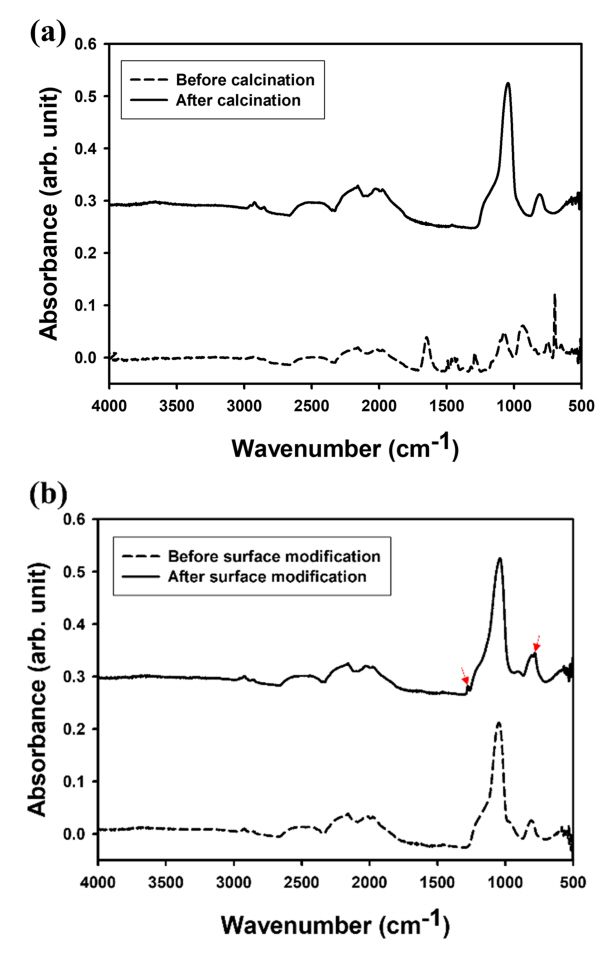
In this study, FT-IR analyses were carried out to confirm successful formation of porous silica fibers and the surface modification of the resulting fibers. A characteristic peak at 1,600 cm-1 originating from the PS nanospheres was detected before calcination, as presented in the spectrum of Fig 4(a) [30]. This peak disappeared after the thermal decomposition of the polymeric particles. In Fig 4(a), the solid line in the FT-IR spectrum of the sample after calcination contains the characteristic peaks of SiO2 at 800 and 1,110 cm-1 [31]. Therefore, it can be concluded that the gelation of TEOS with hydrochloric acid took place in the electro-spun fibers, indicating that the transformation to porous silica was successful after heat treatment at 500 °C [32-34].
To confirm the hydrophobic surface treatment using the silane coupling agent, FT-IR spectra of the porous fibers were obtained before and after the surface modification, as shown in Fig 4(b). The FT-IR analysis shows additional absorption bands at 786 and 1,273 cm-1 due to the MTCS treatment, attributed to the characteristic vibrations of Si–O–Si and C–Si asymmetric stretching present in the C–Si–O units. At the absorption peak around 2,923 and 2,852 cm-1, the intensity becomes sharper after successful MTCS grafting compared with the porous silica fibers, likely due to the attachment of the –CH3 groups of the MTCS [35].
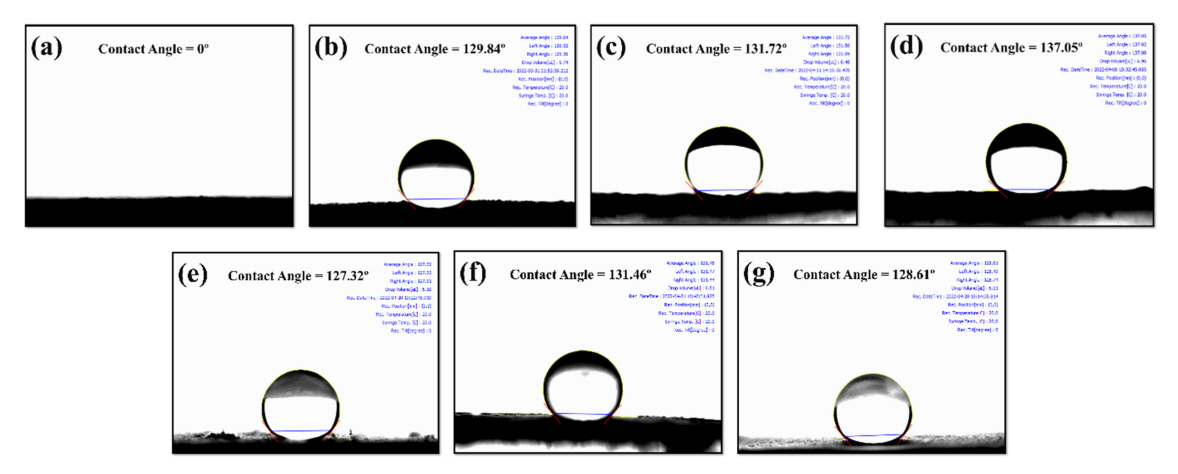
To study the surface properties of the porous silica fibers, a sample was coated on a glass substrate and bonded with adhesive. Before surface modification, the contact angle of a water droplet could not be measured, because the droplet spread out on the surface of the film composed of porous fibers, as shown in the photograph in Fig 5(a). This indicates the hydroxyl groups on the fibers formed by electrospinning were superhydrophilic.
This study investigated the effect of the amount of silane coupling agent on surface modification, by measuring the amount of adsorbed oil as a function of the amount of MTCS. It was determined that 0.042 mol of MTCS (2.1 times) resulted in the maximum amount of adsorbed oil. Therefore, the porous silica fiber was fabricated with 400 nm diameter PS nanospheres and modified with MTCS, PTCS, DTCS, BTCS, OTES, and MPTMS under the same conditions, to measure the contact angle of a water droplet for each case. After calcination, hydrophobic porous silica fiber samples before and after surface treatment were mounted onto glass slides using double-sided adhesive tape. The syringe was programmed to deliver a drop of distilled water (6 to 7 µl) to the surface to record the measured contact angle value. After surface modification with MTCS, PTCS, DTCS, BTCS, OTES, and MPTMS, the measured static contact angle of the water droplet was 129.84, 131.72, 137.05, 127.32, 131.46, and 128.61º respectively, due to the lotus effect (Fig 5 (b), (c), (d), (e), (f), (g)) [36]. Because the silane coupling agent was attached covalently to the silica fibers’ surface containing spherical macropores, hydrophobic molecules were formed, causing water-repellent surfaces.
3.3 Adsorption of oil using the hydrophobic porous silica fibers as adsorbents
Fig 6(a) shows a photograph of the surface-modified macroporous silica fibers with methyltrichlorosilane before oil adsorption, colored gray and brown. A photograph of the surface-modified macroporous silica fibers after oil adsorption is presented in Fig 6(b). Because the silicone was stained with Sudan III, the color of the oil-silica mixture was dark black with a red tint. During the oil adsorption test, oil can enter the silica fibers through holes in the fibers as well as the space between the fibers. When saturation is reached, the oil and silica fiber fuse to form a homogeneous mass with no fluidity.
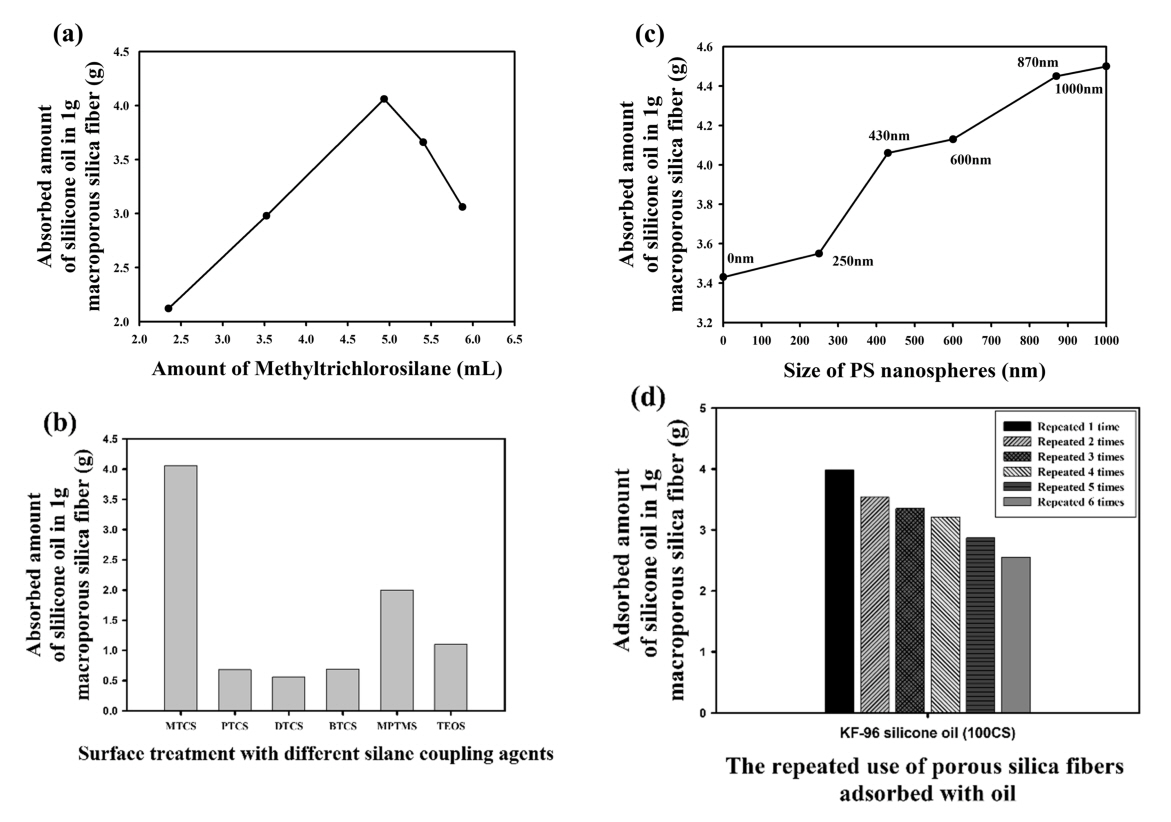
To test whether the amount of oil adsorbed depends on the amount of silane coupling agent, PS nanospheres 430 nm in diameter were used to fabricate macroporous silica fibers. Then, the obtained macroporous silica fibers were surface-modified with MTCS with different amounts of the coupling agent, to compare the amount of oil adsorbed. The results showed that the amount of oil adsorbed depends on the amount of MTCS. It increased continuously from 0.02 mol (1X) to 0.042 mol(2.1X) of MTCS, and then decreased from 2.1X to 2.5X (0.05mol) of MTCS. An excess dosage of MTCS could lead to blocking of the porous structure of silica fibers, and dramatically reduce the amount of oil adsorption. The largest amount of oil adsorption was 2.1X of MTCS, as shown in Fig 7(a).
In this study, comparative oil adsorption tests were also conducted using different silane coupling agents during surface modification. The amount of oil adsorbed by the porous silica fibers fabricated using 430-nm PS nanospheres was measured, depending on the type of silane coupling agents, as shown in Fig 7(b). The porous silica fibers modified with MTCS exhibited the largest oil adsorption capacity, 4.06 g silicone oil per 1 g of the fibers, indicating that it was more advantageous to use MTCS than other silane coupling agents.
Compared with the other coupling agents, MTCS has a simple structure, containing one methyl group. This means that after surface modification, a bonding layer is created on the surface in a moderate and ventilated way, promoting the oil adsorption process. In contrast, with surface modifiers containing dense and complex reactive groups, the silanes coated on the porous silica fibers formed organic films of single molecular thickness. These molecules are attracted to each other and become too tight, forming a thick film that interferes with oil penetration into the porous silica fibers. Because the molecular structures in silane coupling agents other than MTCS are bulky, the existing nano or mesopores can be blocked after surface modification, preventing the oil from penetrating into the pores.
In contrast, with MTCS, a very thin hydrophobic layer can be expected after surface modification, because there is only one methyl group in the MTCS molecule. Factors that can affect the ability to generate hydrophobic surfaces include organic substitution, the extent of surface coverage, remaining unreacted groups, and the distribution of silane on the surface. Surface modification is maximized when the silane reacts with the substrate surface and reaches the maximum number of accessible sites on the surface.
Hydrophilic surfaces are usually polar with distributed hydrogen bonding sites. Accordingly, a successful hydrophobic coating must remove or minimize hydrogen bonding and protect polar surfaces from interaction with water by creating a non-polar interphase. A hydrophobic surface is achieved when all the hydroxyl groups are limited by silanes and the surface is effectively shielded. Experiments performed with different silane coupling agents revealed that MTCS meets these criteria, resulting in the highest oil absorption. This confirms that using MTCS to modify the porous silica fibers is the optimum choice.
Macropores exist on the surface of the porous silica fibers whose size can be controlled using the diameters of the PS nanospheres, and increasing the porosity of the fibers compared to nonporous fibers. In this study, a series of samples of porous silica fibers were synthesized using PS nanospheres with sizes of 250, 430, 600, 870, and 1000 nm in diameter. In addition to porous fibers, silica fibers were also fabricated by electrospinning without PS nanospheres and measured for oil adsorption (nonporous silica fibers). As shown in Fig 7(c), the amount of adsorbed oil increased as the diameter of the PS nanospheres increased, indicating that the macropores enhanced oil adsorption; the measured oil adsorption capacity of the nonporous silica fiber had its lowest value. The oil adsorption capacity of the porous fiber samples synthesized using PS nanospheres with 250, 430, 600, 870, and 1000 nm in diameter increased gradually as a function of the macropore size and reached the maximum value at 1000 nm in diameter. These results confirm that PS nanospheres with a larger size generated macropores with a large diameter and enhanced the adsorption of oil due to the resulting facile infiltration of the liquid contaminant into the larger macropores. This improved the silica fiber’s ability to adsorb oil inside pores, as well as the void spaces between the fibers.
Fig 7(d) shows that the adsorption capacity of the macroporous silica fibers gradually decreased with repeated use. After adsorbing oil, the macroporous silica fibers were washed 2 times using hexane. However, a small portion of oil can remain inside the pores of the fibers, causing a gradual decrease in the amount of oil adsorbed. After 6 recycles, the amount of silicone oil adsorbed by the macroporous silica fibers (430 nm PS) decreased to 2.55 g, as presented in Fig 7(d). The results showed that repeated use of the porous fibers resulted in a slight decrease in oil adsorption efficiency. However, materials could still be reused to save manufacturing costs as well as contribute to environmental protection.
In this study, meso-macroporous silica particles were also prepared for oil adsorption to compare their adsorption capacity with that of the porous silica fibers. The meso-macroporous silica particles were synthesized by the evaporation-driven self-assembly of PS nanospheres and Pluronic F127 as dual templates [37,38]. After calcination, the morphologies of the porous silica particles were observed using an electron microscope, as shown in the SEM image of Fig 8(a). Though only macropores were observed from the spherical particles, smaller mesopores were also formed by structure-directing agents like Pluronic F127.
For oil adsorption, surface modifications were made in a manner similar to those employed for the porous silica fibers. Surface modification with MTCS was carried out with different amounts of MTCS, 0.5X, 1X, 1.5X, 2.1X, 3X, 5X, respectively. The obtained results showed that 0.5X MTCS resulted in the best oil adsorption capacity of 3.25 g of oil per 1 g of meso-macroporous silica particles. The results indicate that the amount of oil adsorbed by the porous silica fibers tended to decrease as the amount of silane coupling agent increased, because smaller mesopores can be blocked by an excessive amount of MTCS, decreasing oil adsorption.
Specifically, after surface treatment with quantities of 1X, 1.5X, 2.1X, 3X, and 5X MTCS, meso-macroporous silica particles showed oil adsorption per gram of porous particles of 2.8, 2.35, 2.17, 2.20, and 2.13 g, respectively, as shown in Fig 8(b). This result shows that the oil adsorption capacity of the macroporous fibers is better than that of the meso-macroporous silica particles and aerogel powders synthesized in our previous study [39]. This is because it provides additional void space between silica fibers for oil adsorption, which can contain additional oil.
4. CONCLUSIONS
Macroporous silica fibers were synthesized by electrospinning using PS nanospheres as templates to fabricate macropores in the fibers. The amount of oil adsorbed per gram of fiber was controlled by adjusting the diameter of the PS nanospheres used as templates, and changing the silane coupling agents or the amount of coupling agent used for surface modification. The pore size that appeared on the fibers’ surface after calcination increased as the diameter of the PS nanospheres increased. The amount of oil adsorbed increased after the surface treatment and increasing size of macropores, because the infiltration of oil through smaller pores is difficult. It was concluded that the amount of oil adsorbed is proportional to the diameter of the macropores in the porous fibers. The surface modification to transform surface hydroxyl groups into hydrophobic functional groups of silane coupling agents is an important step for the adsorption of oil. The resulting porous silica fibers were film coated, and their water-repellent property was confirmed by measuring the static contact angle of water droplets. The experimental results confirmed that the oil adsorption capacity of the porous silica fibers after surface modification with 0.042 mol (2.1X) of MTCS resulted in the highest amount of oil adsorbed. In addition to the porous fibers, the oil adsorption capacity of meso-macroporous silica particles was measured. The macroporous silica fibers exhibited better results, because the void spaces between silica fibers adsorbed additional oil.