1. INTRODUCTION
Gold is a highly valued metal with excellent chemical and electrical properties, making it a popular choice for use in jewelry and electronic devices [1,2]. As the demand for gold, it has become increasingly important to recover it from secondary resources. The first step in the recovery process is to dissolve the gold present in the scrap. Sine the oxidation potential is very low of gold this step requires the use of oxidizing agents in HCl solutions. Several separation methods have been employed to recover Au(III) from the leaching solutions, including solvent extraction [3,4], ion exchange [5], reduction [6], and precipitation [7]. Among these methods, solvent extraction is the most effective and cost-efficient method for separating Au(III) from solutions containing other metal ions.
Gold (III) in concentrated hydrochloric acid solutions forms AuCl4− when the concentration of chloride ions is higher than 0.1 M [8]. To selectively separate gold (III) from other metal ions in HCl solutions, various types of acidic (Cyanex 272 and LIX 63), neutral (TBP, DBC and MIBK), and basic (Aliquat 336) extractants have been used [9-12]. However, the selectivity of TBP, MIBK and Aliquat 336 for gold (III) from concentrated HCl solutions is not good, resulting in low gold (III) from other metal ions due to the co-extraction of impurities. In general, noble metal ions present in the etching solutions of printed circuit boards and electroplating solutions can be separated from other base metal ions by cementation with copper metal [13]. In this case, it is necessary to separate gold(III) ions from the leaching solutions of the cemented copper, which contain other noble metal ions. According to our studies on the solvent extraction of Au(III), Cyanex 272 is the most effective among the above-mentioned extractants in separating Au(III) from the HCl solutions containing Pt(IV) and Pd(II) [8,11,14].
Some data on the solvent extraction of Au(III) by Cyanex 272 have been reported but the extraction reaction has not been identified. McCabe-Thiele diagram is generally employed to predict the number of stages necessary to extract certain amount of target metal ions in multi-stage counter-current extraction. The extraction isotherm can be obtained by either experiments or calculation. In order to calculate the extraction isotherm, extraction reaction together with its equilibrium constant is necessary. In this work, the extraction reaction of Au(III) by Cyanex 272 from HCl solutions was identified and its equilibrium constant was estimated.
For this purpose, the extraction data was obtained by varying the concentrations of Au(III), HCl and Cyanex 272. Slope analysis method was applied to the extraction data in order to propose the extraction reaction. Moreover, Fourier-transform infrared spectroscopy (FT-IR) was compared between fresh and loaded Cyanex 272 to investigate some change in its chemical structure during extraction. Nuclear magnetic resonance (NMR) spectroscopy was also analysed to investigate the molecular level interactions between Cyanex 272 and the extracted Au(III) ions.
2. EXPERIMENTAL
2.1 Chemicals and Reagents
To prepare the synthetic solutions of Au(III), HAuCl4 (Sigma-Aldrich, 30wt% in dilute HCl) was dissolved in hydrochloric acid solutions (Daejung Co., 35%). Cyanex 272 (Solvay Cytec Industries, 85%) was used without purification, while reagent grade kerosene (Daejung Co.) and chloroform-D (CDCl3, Sigma-Aldrich, 99.8%) served as diluents. Analytical grade reagents were used for all experiments. H3PO4 (Daejung Co., 85%) was used as a standard in the analysis of the MNR spectra measurement. All reagents used were of analytical grade.
2.2 Procedure
The extraction experiments involved shaking organic and aqueous phases in a 100 mL screwed cap bottle using a Burrell wrist action shaker (model 75, USA) at ambient temperature (22 ± 1°C) for 30 minutes. The phase ratio of the two phases was fixed at unity. After the reaction, the mixed solutions were left on a separatory funnel to separate the aqueous and organic phases. The concentration of Au(III) in the aqueous solution before and after extraction was measured by ICP-OES (Inductively coupled plasma-optical emission spectrometry, Spectro Arcos, Germany). The concentration of Au(III) extracted into Cyanex 272 was calculated using mass balance. The extraction percentage [15] of Au(III) (%E) was defined by Eq. (1).
where eo and er are the mass of Au(III) ions in the aqueous solution before and after extraction, respectively. The distribution coefficient (D) of Au(III) by Cyanex 272 was calculated as the ratio
where [Au(III)]org and [Au(III)]aq are the total concentration of Au(III) in the organic and aqueous phases after extraction.
The FT-IR spectra of Cyanex 272 was analyzed by FT-IR Spectrometer (Vertex 80V, Bruker) [16] with the method of attenuated total reflectance (ATR). NMR spectra measurements of Cyanex 272 were performed on an ECZ-500 (Broker, JEOL) at room temperature and at 600 MHz for 31P. For the 31P measuring, CDCl3 was used as a diluent, while H3PO4 was used as an internal chemical shift reference to indicate the difference in the resonance frequencies of the atoms [17].
3. RESULTS AND DISCUSSION
3.1 Effect of HCl and Cyanex 272 concentration on the extraction of Au(III)
Au(III) is considered a hard acid and thus has a strong tendency to form complexes with chloride ions. As a result, in HCl solutions where the concentration of HCl is higher than 0.1 M, Au(III) is present as Cl4− [8,18]. Various studies have reported that Caynex 272 can selectively extract Au(III) from concentrated HCl solutions over other metal ions [8,13,14]. Since Cyanex 272 is a weak organic acid, it would not be dissociated in concentrated HCl solutions. Therefore, Cyanex 272 would take part in the extraction of Au(III) as a molecule from concentrated HCl solutions.
Slope analysis method is generally employed to identify the extraction reactions. In order to apply solpe analysis method, equilibrium data on the extraction of the metal ions are necessary. Therefore, the effect of Au(III), HCl and Cyanex 272 concentrations on the extraction of Au(III) was investigated. First, Au(III) solutions with concentrations of 0.1, 0.5 and 1.0 g/L were prepared by varying the concentration of HCl in the solutions from 1 to 9 M.
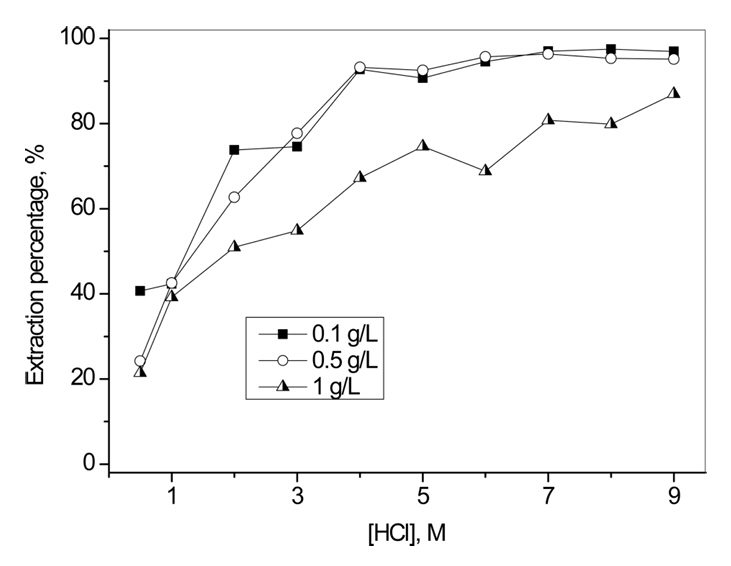
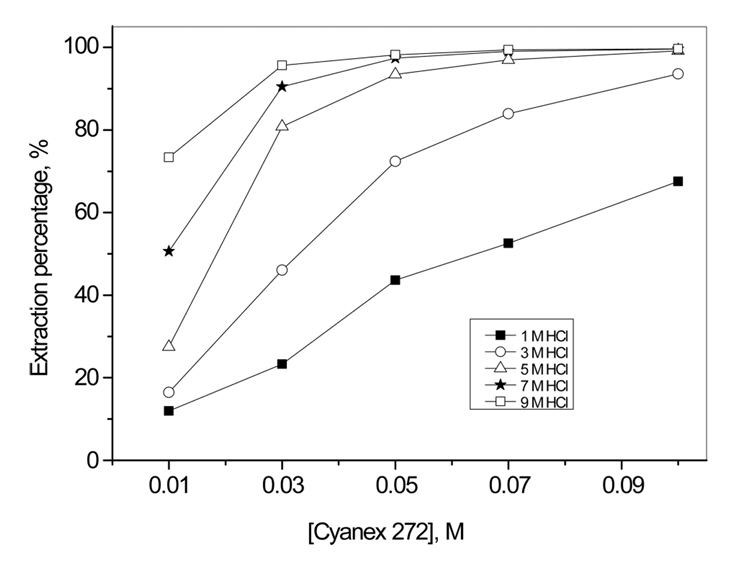
Fig 1 represents the variation in the percentage of Au(III) extraction with HCl concentrations, for several Au(III) concentrations, using 0.05 M Cyanex 272. The extraction percentage increased quickly when HCl concentration increased from 1 to 5 M, then slowly increased as HCl concentration increased to 9 M. Additionally, the extraction percentage of Au(III) was affected by the initial concentration of Au(III), and was at its minimum when Au(III) concentration was 1.0 g/L. In Fig 2, it is clear that the dependence of Au(III) extraction on Cyanex 272 concentration was affected by HCl concentration. The extraction percentage of Au(III) became higher when HCl concentration increased from 1 to 9 M. Furthermore, when HCl concentrations were 1 and 3 M, the extraction percentage of Au(III) was almost linearly increased with Cyanex 272 concentration. However, when HCl concentrations were 5, 7 and 9 M, lower concentration of Cyanex 272 was sufficient to extract most of the Au(III).
Fig 2 clearly indicates that HCl concentration is an important variable in the extraction of Au(III) by Cyanex 272 from HCl solutions.
3.2 Application of slope analysis method to the extraction data of Au(III) by Cyanex 272
Cyanex 272 would not dissociate in concentrated HCl solutions. Therefore, Cyanex 272 molecule would react with Cl4− from concentrated HCl solutions. According to our previous work [13], Cyanex 272 can completely separate Au(III) from other metal ions such as Pd(II) and Cu(II) present in an HCl solution. Moreover, Xing et al. [8,9] also reported that Cyanex 272 can selectively extract Au(III) over either Pd(II) and Pt(IV) or Zn(II) and Ni(II) from the HCl solutions. When Au(III) is extracted by Cyanex 272 through solvating mechanism, electrically neutral species of Au(III) can be extracted. There might be two kinds of electrically neutral species of Au(III) in HCl solutions, AuCl3 and HAuCl4 which can be formed by the interaction between AuCl4 and hydrogen ions. Therefore, the extraction reaction of Au(III) by Cyanex 272 through solvating mechanism can be represented as the following two equations.
where n represents the number of moles of Cyanex 272 (HA) that takes part in the reaction.
Our extraction results indicated that the extraction percentage of Au(III) was increased with HCl concentration. Considering that the mole fraction of AuCl3 would be decreased with HCl concentration and hydrogen ions take part in the extraction reaction of Cl4−, Eq. (4) can better represent the extraction reaction of Au(III) by Cyanex 272 from HCl solution through solvating mechanism. After inserting the definition of the distribution coefficient of Au(III) into the equilibrium constant of Eq. (4) and rearrangement of the resulting equation would lead to the following equation.
and [H+] and [HA] represent the equilibrium concentration of hydrogen ion and Cyanex 272 after extraction. The equilibrium concentrations of the reactants can be calculated by using the mass balance as follows
In the above equations, subscript initial means the initial concentration. When the initial concentrations of HCl and Cyanex 272 are in excess of that of Au(III), the equilibrium concentrations of hydrogen ion and Cyanex 272 might be equal to the initial concentrations of hydrogen ion and Cyanex 272.
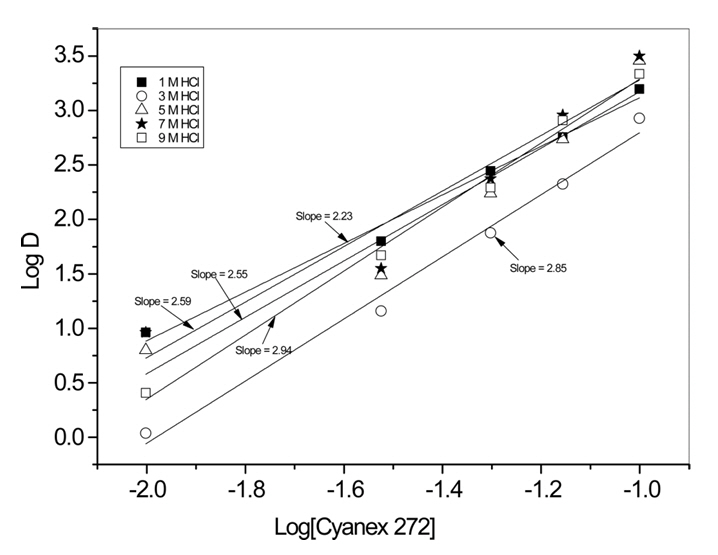
Fig 3 illustrates the logarithmic plot of HCl concentration against the logarithmic distribution coefficient of Au(III), with a fixed Cyanex 272 concentration of 0.05 M. All three concentrations of Au(III) had a slope of one, which matches Eq. (5). This result indicates that one mole of hydrogen ions is required to extract one mole of Au(III). On the other hand, Fig 4 depicts the logarithmic plot of Cyanex 272 concentration against the logarithmic distribution coefficient of Au(III) for various HCl concentrations, with a fixed initial concentration of Au(III) at 0.1 g/L. The slopes of the plots were between 2.2 and 2.9, indicating that at least two moles of Cyanex 272 are necessary to extract one mole of Au(III).
3.3 Determination of the loading capacity of Cyanex 272 for Au(III)
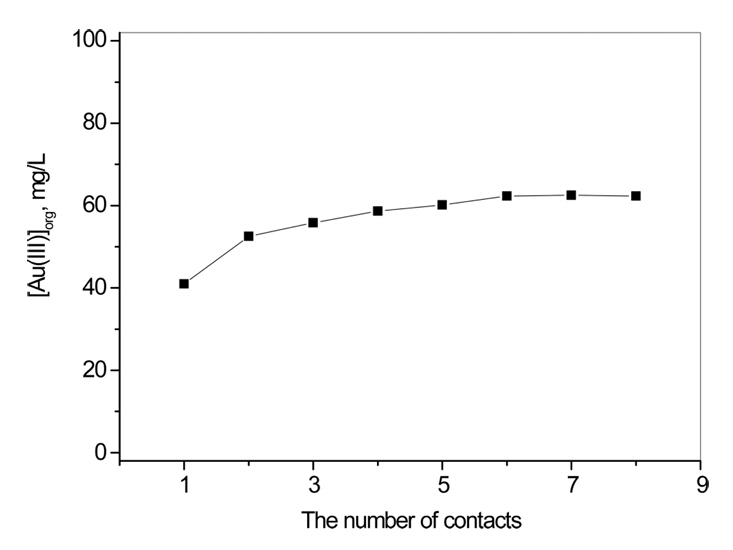
The slopes of the plots in Fig 4 were in the range between 2.2 and 2.9. Therefore, in order to determine the number of moles of Cyanex 272 participating in the extraction of one mole of Au(III), loading capacity of Cyanex 272 for Au(III) was measured. For this purpose, 0.01 M Cyanex 272 was consecutively contacted with 0.1 g/L Au(III) solution until the Au(III) in the loaded organic was saturated. In these experiments, the concentration of HCl was fixed at 5 M.
Fig 5 shows the cumulative concentration of Au(III) in the loaded Caynex 272 after each stage of contact. After 7th stage, Au(III) concentration in the loaded Cayenx 272 was saturated. The loading capacity of 0.01 M Cyanex 272 was determined to be 0.07 g/L Au(III). At the loading capacity of Cyanex 272, the molar ratio of Cyanex 272 to Au(III) was about 30, indicating that molecular HCl can be extracted by Cyanex 272. Since the mole fraction of molecular HCl would be proportional to HCl concentration, it might be said that the loading capacity of Cyanex 272 for Au(III) would be decreased with HCl concentration.
Based on the slope analysis of the extraction data and loading capacity of Cyanex 272, the following reaction was proposed as the extraction reaction of Au(III) by Cyanex 272 from HCl solutions.
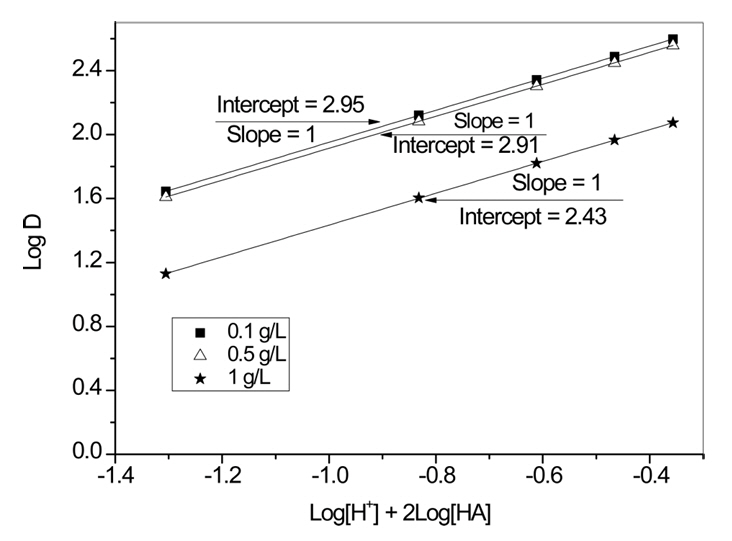
Eq. (8) indicates that the plot of log [H+] + 2log [HA] vs. log D would result in a straight line with a slope of unity.
Fig 6 represents these plots for several concentrations of Au(III). The values of the slope of these plots were unity, verifying that Eq. (8) is responsible for the extraction of Au(III) by Cyanex 272 from HCl solution. The intercept of Fig 6 is equal to the equilibrium constant of Eq. (8). The equilibrium constant of Eq. (8) was estimated to be 2.7 by taking the mean of the intercept values of Fig 6.
3.4 Analysis of FT-IR spectra data
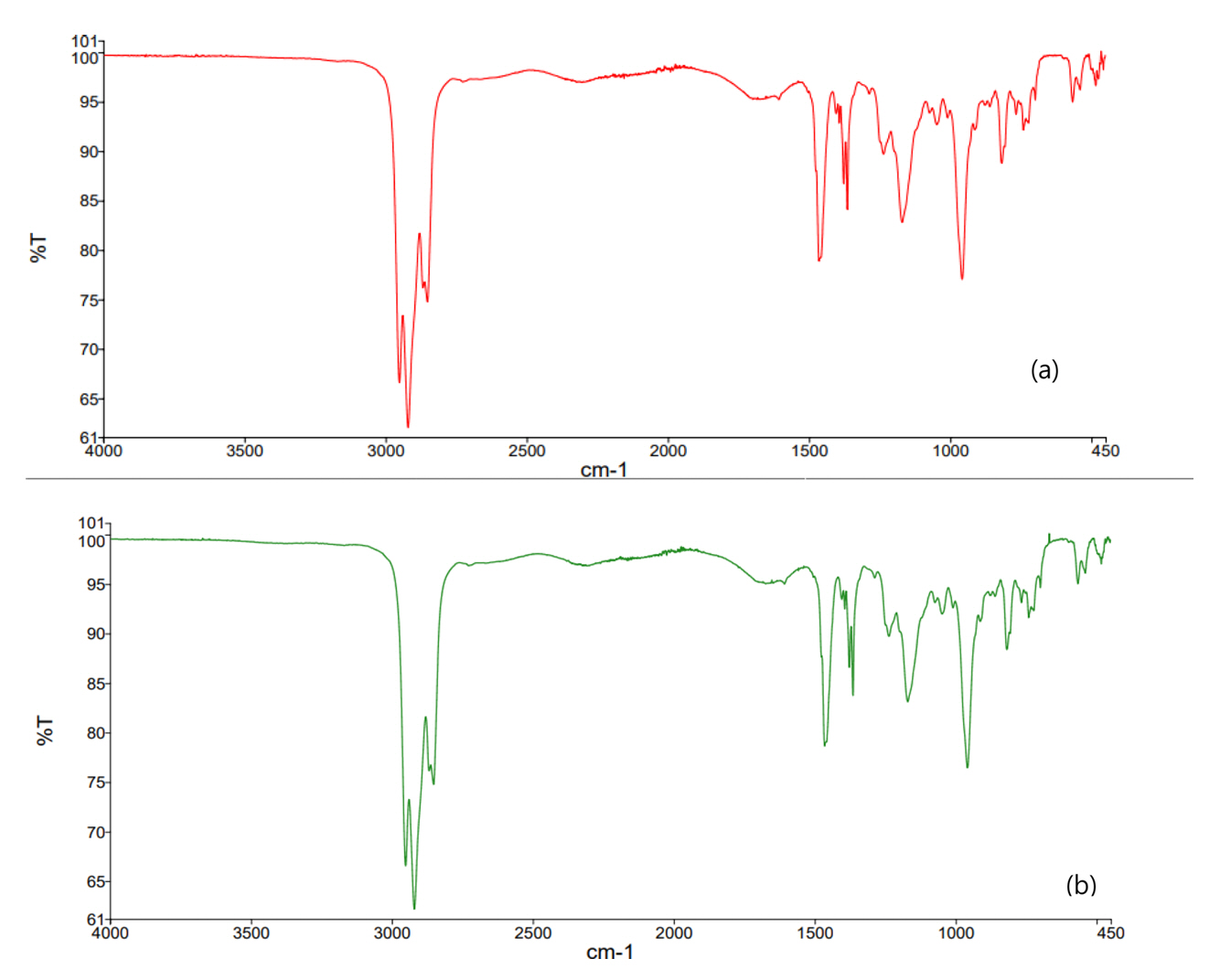
There might be some change in the FT-IR of Cyanex 272 when some reactions occur at the phosphinic acid of Cyanex 272. In this case, it is necessary to investigate the specific vibrational characteristic bands of Cyanex 272 before and after the reaction. Therefore, the FT-IR of fresh Cyanex 272 and loaded Cyanex 272 was compared and is represented in Fig 7.
Table 1 lists the characteristic vibration bands of the fresh Cyanex 272 and the loaded Cyanex 272. The absorption bands at 2953.8-2870.8 cm−1 are assigned to the hydrogen bond of the dimeric Cyanex 272. The absorption band at 1465 cm−1 corresponds to the P-O stretching vibration of fresh Cyanex 272. This P-O vibration band shifts to little higher wave number at 1466 cm−1 for the loaded Cyanex 272. This might indicate the occurrence of coordination reactions between the P-O group and the extracted Au(III). To investigate this kind of interaction, NMR spectra will be analysed in next section. Meanwhile, the characteristic peak of P-O-C in the fresh Cyanex 272 was 1170.6 cm-1, which was nearly same to the P-O-C peak (1170.5 cm-1) of the loaded Cyanex 272. The intensities of the P-O-H stretch band at 957.8 cm-1 of the loaded Cyanex 272 was almost similar to that of the fresh Cyanex 272 (957.9 cm-1) [19-21].
There was negligible change in the characteristic vibration bands of P=O, P-O-C and P-O-H between fresh and the loaded Cyanex 272, indicating that there was no change in the chemical structure of Cyanex 272 during the extraction of Au(III) from HCl solutions. Therefore, it can be said that Cyanex 272 take part in the solvent extraction of Au(III) as a molecule.
3.5 Analysis of NMR spectra data
A characteristic of organophosphorus acidic extractants like Cyanex 272 is that they form stable dimer in non-polar solvents [22]. When the concentration of chloride ion is high, metal chloride complexes can be extracted by organophosphorus acidic extractants through either outer sphere assemblies or ion pairs as represented in Eq. (8). The phosphinic acid (-PO2) group of Cyanex 272 can form coordinate bond with gold ions. A single Cyanex 272 molecule has several coordination sites, allowing it to coordinate up to four gold ions simultaneously. This results in the formation of complex with tetrahedral geometry which is stable due to the optimal overlap of orbitals and efficient sharing of the electron pairs between Cyanex 272 and the gold ion [23].
The molecules or atoms absorb the electromagnetic waves of certain energy in a specific external magnetic field, reflecting the structural information of the molecule of interest [24]. To determine the chemical shifts of the P atoms, 31P NMR spectroscopy was employed.
Figs 8(a) and (b) display the 31P NMR spectra of fresh and loaded Cyanex 272. The spectra of fresh Cyanex 272 shows large resonances at 50.8 and 60.4 ppm for the phosphorus atom, and small peaks at 35.4, 4.6, and 0.5 ppm, which might be ascribed to the presence of some impurities. However, when Au(III) was extracted into Cyanex 272, the signals of the phosphorus atoms at 50.8 and 60.4 ppm disappeared in the complex spectra. This indicates that Au(III) ions form coordination bonds with the phosphorus atom of Cyanex 272 [25-27]. The FT-IR spectra showed that the chemical structure of Cyanex 272 remained unchanged during the extraction of Au(III). Based on the results obtained from FT-IR and NMR spectra, it can be said that the extraction mechanism of Au(III) by Cyanex 272 occurs through the solvating mechanism, and Au(III) ions form coordinative bonds with the phosphorus atom of Cyanex 272.
4. CONCLUSIONS
The extraction reaction of gold(III) from hydrochloric acid solution by Cyanex 272 was studied and identified. Among several variables like the concentrations of Au(III)(0.1-1 g/L), HCl(1-9 M) and Cyanex 272(0.01-0.1 M), the effect of HCl concentration on the extraction of Au(III) was the most pronounced. The extraction percentage of Au(III) was increased as the concentration of HCl and Cyanex 272 increased. By applying slope analysis method to the extraction data, the extraction reaction of Au(III) by Cyanex 272 from concentration HCl solution was proposed as
Cl4− (aq) + H+ (aq) + 2HA(org) = [HAuCl4·2HA](org)
This reaction agreed well with the extraction data that the extraction of Au(III) increased with the increase of HCl concentration. The loading capacity of Au(III) by 0.01 M Cyanex 272 was determined to be 0.07 g/L, indicating that Cyanex 272 extract both electrically neutral Au(III) complexes and HCl. FT-IR spectra showed no significant change in the functional groups of Cyanex 272 during Au(III) extraction. NMR spectroscopy revealed a change in the characteristic peaks of the phosphorus atom of Cyanex 272, indicating the formation of a coordinative bond between Au(III) and Cyanex 272.