1. INTRODUCTION
Fine metallic nanopowders have many advantages, including high hardness, toughness, and strength, and as a result have been intensively investigated and used extensively as raw materials in various industrial fields, including spaceflight, electronics, national defense, and engineering. Among various metallic nanopowder systems, tungsten (W) nanopowder has attracted great interest as a potential candidate for catalysis, hard materials, thermionic cathodes, and high-power batteries, because of its highly oxophilic characteristics [1]. However, at present, the production of metallic W powder is a complex multistep process involving the processing of W ore concentrates, in the following stages: (i) Separation of useful components from the W ore, (ii) Isolation of ammonium paratungstate or other compounds, (iii) Purification by reduction and finally, (iv) Metallic W production [2]. Compared with the several operational steps required for the production of W powders, a facile synthesis process to produce metallic W nanopowder would be an important development.
Nano-sized W powder synthesis is typically performed using one of two different approaches, top-down or bottomup routes. Approaches include mechanical milling [3-5], thermal decomposition [6], molten salt electrolysis [7], solution combustion synthesis [8], epitaxial growth [9], and chemical reaction [10]. However, due to a resistance to acids and alkalis, it is hard to produce W nanopowders, along with common techniques such as sol-gel, hydrothermal, chemical reaction, and conventional evaporation/condensation. Furthermore, the preparation of metallic W nanopowder requires substantial retooling of conventional processes, making the materials relatively costly and not amenable to mass production.
As an alternative method, the thermal plasma technique may be a suitable choice, and has been used by several authors for the synthesis of metallic W nanopowder [11-14]. The thermal plasma technique has several advantages to provide the materials with high temperature, good thermal and electrical conductivity, and other exotic properties and has been studied in a variety of applications, especially for the synthesis of various functional nanomaterials [15-19].
Among the different types of thermal plasmas, such as direct-current (DC) plasma, radio-frequency (RF) induction plasma (or inductively coupled plasma) has the unique advantages of a relatively large plasma volume, a lower plasma velocity, clean high-energy, and good chemical reactivity. RF induction thermal plasma is especially suitable for materials with high melting points because of its long residence time, and has been extensively used for the synthesis of ultrafine powders, spheroidization, and deposition [11, 12, 14, 17-22]. Krasovskii et al. reported the production of alpha-W (α-W) in a DC plasma reactor from an oxide feedstock [13]. Ryu et al. reported the production of W nanopowder with a particle size (d50) of 88.5 nm using a thermal plasma process with ammonium paratungstate (APT) [12]. Sarmah et al. reported a W nanopowder synthesis using a plasma expansion technique with W trioxide (WO3) in which H2 gas was used as a reductant [11]. Enneti et al. investigated the synthesis of nanocrystalline W in a single step via a thermal plasma technique [14]. Elsewhere, we have reported the synthesis of nanopowder by RF induction plasma using three different precursor materials, commercial tungsten, tungsten trioxide, and APT [23].
W has both a stable alpha-W (α-W) phase and metastable beta-W (β-W) phase. The α-W phase has a body-centered-cubic (bcc) structure and is highly preferred because it exhibits better properties (e.g., higher electrical conductivity, and better mechanical strength) than the β-W phase [24]. The β-W phase has a primitive cubic (A15) and a non-equilibrium crystalline structure. Although the α-W phase is preferred to the β-W phase, the formation of mixed-phase α- and β-W nanostructures have been frequently reported in the synthesis of W-based nanoparticles and thin films [11, 12, 17, 25-28], indicating that the preparation of α-W single-phase nanostructures is not easy.
In our current study, single-phase α-W nanopowders were synthesized from commercial APT powder in a two-stage process. First, WO3 nanopowder was synthesized by RF induction thermal plasma treatment of the APT. Secondly, the WO3 nanopowder was annealed in an H2 atmosphere to reduce it to a single-phase α-W nanopowder. One should not confuse the present investigation with our research reported elsewhere [23]. The previous research compared three different precursors for synthesis and explored the possibility of single-phase α-W nanopowder synthesis. Our current investigation utilizes two findings from the prior study ((i) that APT in the absence of H2 produces WO3 powder and (ii) WO3 powder under H2 atmosphere can produce W nanopowders) to synthesize single-phase α-W nanopowder. The present process is completely dry and was carried out without any chemicals except Ar and H2.
The characteristics of the synthesized nanopowders were investigated using X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), and transmission electron microscopy (TEM). Although a few metallic single-phase α-W nanostructures have been reported, to the best of our knowledge, the preparation of a single-phase α-W nanopowder via such a simple process (i.e., a combination of thermal plasma treatment and thermochemical reduction) has not yet been reported. Moreover, the particle size of the single-phase α-W nanopowder was easily controlled by the annealing temperature in the thermochemical reduction of the WO3 nanopowder. The novelty of the current research is summarized below.
(i) Problems reported in earlier research [23], like mixed-phase W nanopowder synthesis, were overcome.
(ii) Utilizing commercially available APT, and a two-step RF induction thermal plasma treatment process, pure single-phase α-W nanopowder can be synthesized for commercial applications.
(iii) An important material process engineering challenge has been successfully addressed through the current investigation.
2. Experimental
2.1. Materials
Commercial ammonium paratungstate (APT; (NH4)10H2 W12O42(H2O)4, Ganzhou Grand Sea W&Mo Group Co., LTD) powder and commercial WO3 powder (Ganzhou Grand Sea W&Mo Group Co., LTD) was used without further purification as the precursors for the synthesis of single-phase α-W nanopowder. Ar (99.999% purity) was used as the central gas, sheath gas, carrier gas, and quenching gas in the RF induction thermal plasma system. Whenever needed, H2 (99.999% purity) was used along with Ar as the reducing agent.
2.2. Configuration of the RF induction thermal plasma system
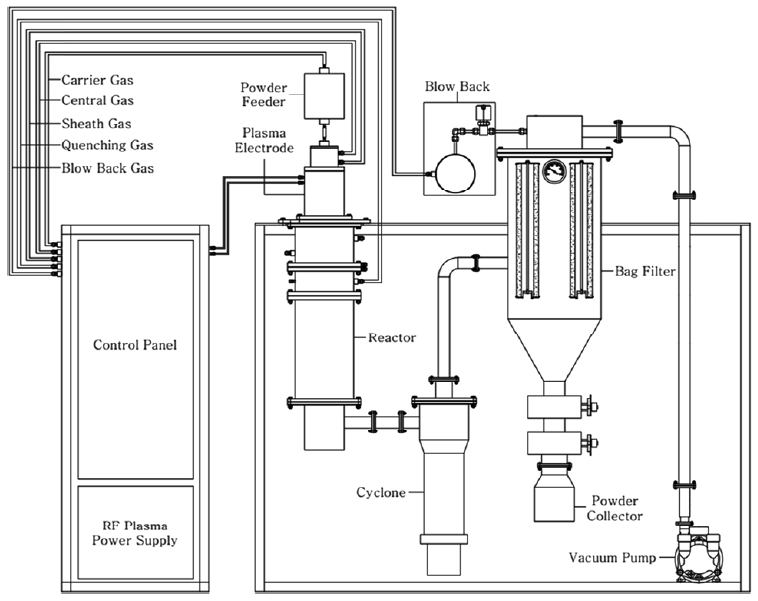
The RF induction thermal plasma system consisted of an RF power supply (30 kW, 4 MHz), a plasma torch, a reactor chamber, a powder-feeding system, and powder-collecting sintered-metal filters. A more detailed configuration of the RF induction thermal plasma system is provided in Fig 1.
2.3. Synthesis of single-phase α-W nanopowder
Single-phase α-W nanopowder was synthesized in a two-stage process through RF induction thermal plasma treatment using ATP as a precursor. Initially, in the first step of the RF induction thermal plasma treatment process, the precursor APT was injected into the RF induction thermal plasma under the experimental conditions listed in Table 1. In the table, “Sheath gas of 50 SLPM (Ar) + 0 SLPM (H2)” corresponds to Ar sheath gas without H2. Before the precursor was injected, the reactor chamber was purged with Ar for 30 min and pre-conditioned to the plasma power as indicated in Table 1 for 20 min, until the system reached a steady level. The precursor particles were carried into the plasma flame with the carrier gas by the powder-feeding system, vaporized by the plasma flame, and quenched rapidly by the quenching gas to accelerate the nucleation kinetics of the nanoparticle synthesis. The synthesized nanoparticles were collected using powder-collecting sintered-metal filters (pore size = 1 μm). In the second step, the thermochemical reduction process, the nanopowder synthesized in the first step by RF induction thermal plasma was annealed in an H2 reductive atmosphere. The conditions for H2 reductive annealing were an H2 flow rate of 0.5 L/min, Temperature of 600-900 °C and 10 min process time.
2.4. Characterization
The morphology of all the as-synthesized nanopowders was analyzed using field emission scanning electron microscopy (FESEM; JSM-6700F, JEOL, Japan) and high-resolution transmission electron microscopy (HRTEM; JEM-2100F, JEOL, Japan) along with energy dispersive X-ray spectrometry (EDX). The phase compositions of the nanopowders were analyzed using X-ray diffraction (XRD; XRD-6100F, Shimadzu, Japan), and their particle sizes were determined from their FESEM images. The thermal characteristics of the APT precursor were analyzed by thermogravimetric/differential thermal analysis (TG/DTA; DTG-60H, Shimadzu, Japan) in an Ar atmosphere.
3. RESULTS AND DISCUSSION
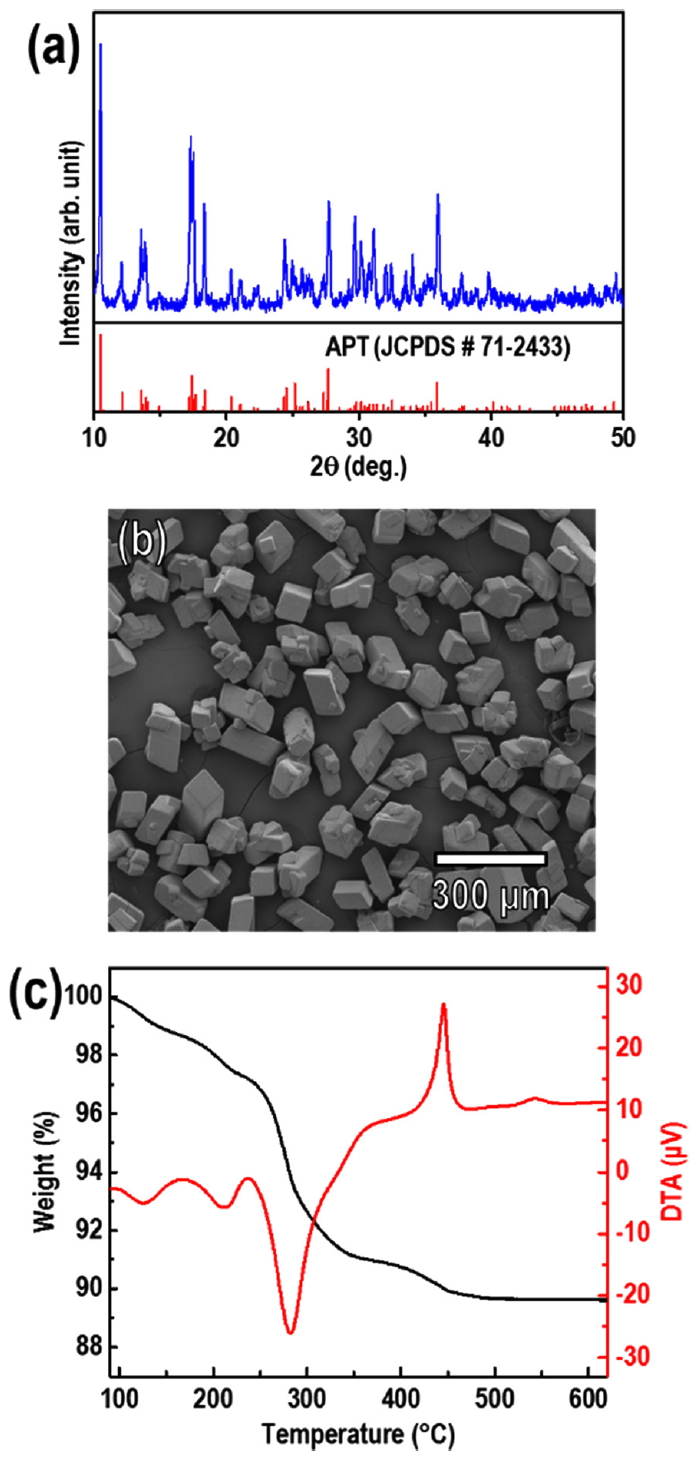
The crystallinity, morphology, and thermal characteristics of the APT precursor were analyzed using XRD, FESEM, and TG/DTA, as shown in Fig 2. The XRD pattern of the APT was indexed to monoclinic APT (JCPDS No. 71-2433), the APT consisted of microsized particles (micro-particles) with an average particle size (median diameter, d50) of 83.85 μm. The TG/DTA results showed that the complete decomposition of APT and crystallization as WO3 occurred before the temperature reached 600 °C. This result is in good agreement with prior reports about the thermogravimetric analysis of APT [12, 17, 29, 30], which indicated that the decomposition process mainly consisted of the loss of H2O and NH3 before reaching 500 °C, and fully crystallized WO3 phase was formed. In a typical RF induction thermal plasma system, the temperature of the plasma flame reaches up to 12,000 K and thus is sufficient for the decomposition of the APT precursor.
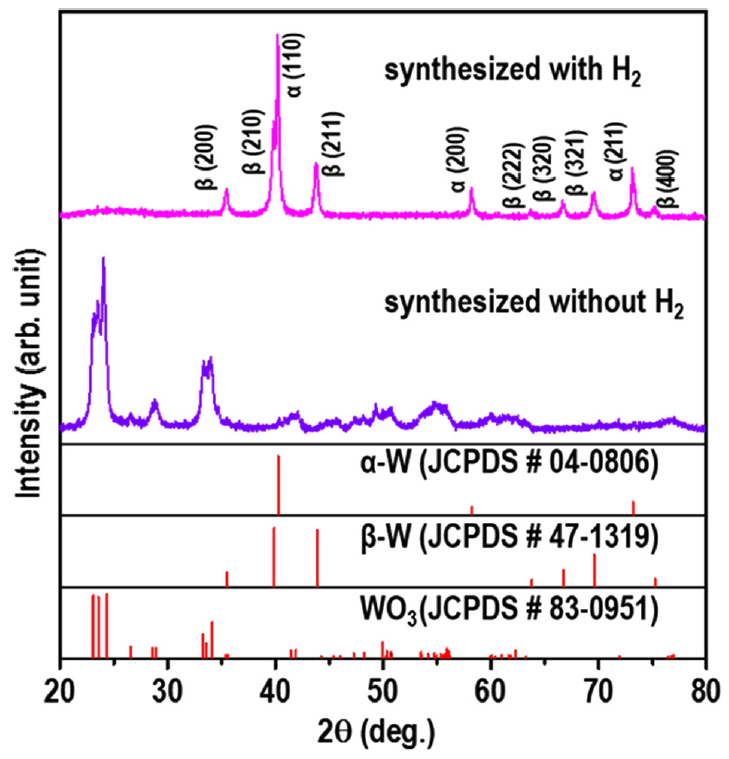
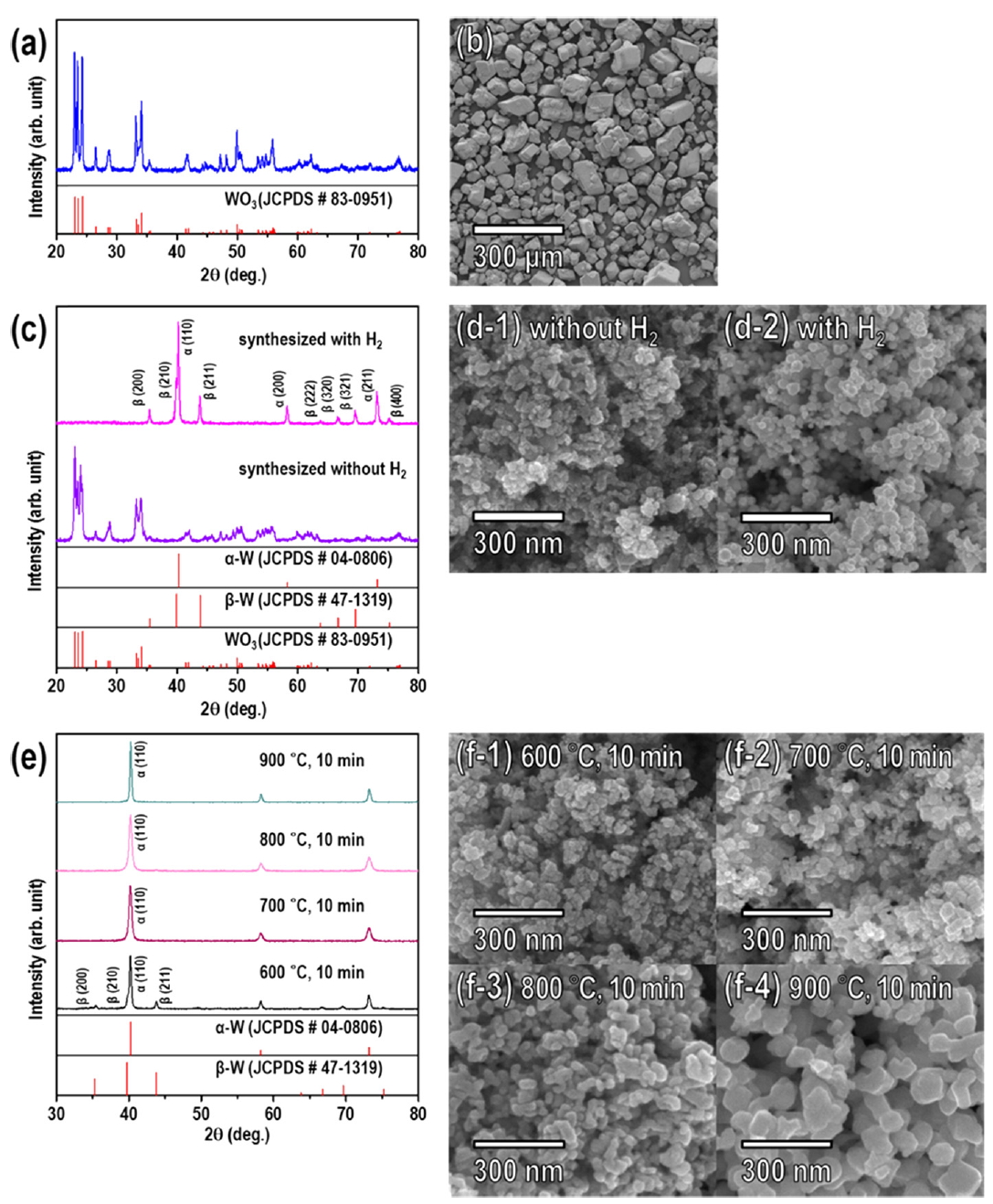
The phase compositions of the powders synthesized from the APT precursor by RF induction thermal plasma with and without H2 are presented in Fig 3. The XRD peaks of the powder synthesized without H2 (Ar only, inert atmosphere) were indexed to monoclinic WO3 (JCPDS No. 83-0951). When APT was injected into the high-temperature jet zone of the RF induction thermal plasma, it was thermally decomposed and evaporated immediately to produce the WO3 powder in an inert atmosphere (Ar). This is a typical plasma-enhanced chemical vapor reaction.
The XRD peaks of the powder synthesized in the presence of H2 (Ar atmosphere with 10% H2) were indexed to a mixture of the α-W (JCPDS No. 04-0806) and β-W (JCPDS No. 47-1319) phases. Thus, the addition of the reducing agent H2 to the Ar atmosphere caused the mixed-phase α-and β-W powder to be produced rather than WO3. The powder consisted mainly of the stable α-W phase with the metastable β-W phase as the minor phase. The ratio of the fraction of the β-W phase to the α-W phase in the W powder was calculated as the diffraction intensity ratio (Rβ-W) of the main peak of the β-W phase, β-W (210) (Iβ-W), to that of the α-W phase, α-W (110) (Iα-W) [9]:
The Rβ-W of the W powder was calculated using Equation (1) and the value was 0.35, indicating that the α-W phase comprised 74% of the W powder.
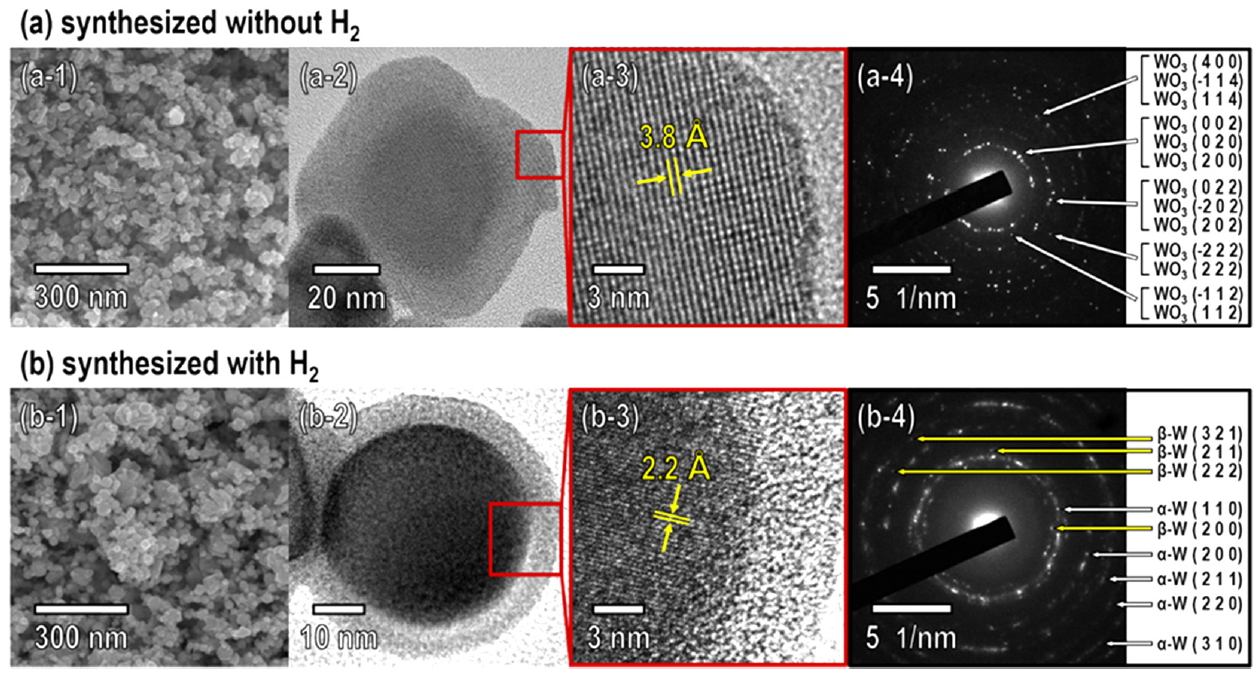
The morphology and crystallinity of the WO3 and W powders synthesized using RF induction thermal plasma was investigated using FESEM, HRTEM, and selected area electron diffraction (SAED), as shown in Fig 4. The WO3 powder synthesized without H2 consisted of nanoparticles with a particle size (d50) of 22.10 nm; the nanoparticles had irregular shapes without an outer shell (Fig 4a-1 and a-2). The HRTEM image corresponding to the region marked by the red square in Fig 4a-2 shows that individual WO3 nanopowder particles had a single-crystalline phase (Fig 4a-3). The measured lattice spacing of 3.8 corresponds to the d-spacing between the (002) planes of WO3 (Fig 4a-3). The SAED pattern shown in Fig 4a-4 also confirmed that the nanopowder exhibited the monoclinic WO3 phase, which was consistent with the XRD results (Fig 3).
The W powder synthesized in the presence of H2 consisted of nanoparticles with a particle size (d50) of 20.34 nm and approximately spherical shape with a core-shell morphology (Fig 4b-1 and b-2). The HRTEM image corresponding to the region marked by the red square in Fig 4b-2 showed a measured lattice spacing of 2.2 , which corresponds to the d-spacing between the (110) planes of α-W (Fig 4b-3). The SAED patterns shown in Fig 4b-4 confirmed that the nanopowder consisted mainly of the α-W phase with β-W as the minor phase, which was consistent with the XRD results (Fig 3).
One explanation for why the β-W phase formed along with the α-W phase when W nanoparticles were synthesized with H2 (Fig 3 and Fig 4b) is that impurities, especially oxygen, can promote the formation of the β-W phase during nucleation and growth [20, 31, 32]. The transformation from the β-W phase into the α-W phase is thermally activated; the oxygen impurities diffuse away and long-range ordering occurs [25]. The presence of the β-W phase in the W nanopowder is proof of the incomplete reduction of the W oxides, or recombination of W and oxygen impurities during the process [20]. This could be because there was insufficient time for the thermal decomposition and reduction of the APT precursor particles in the high-temperature jet zone of the RF induction thermal plasma.
Some other reports have also explained that there is a higher chance of forming the β-W phase with a smaller W nanoparticle grain size [33, 34]. If so, synthesizing W nanoparticles by RF induction thermal plasma is attended by a high chance of forming the β-W phase. Thus, a posttreatment to transform the β-W phase of the W nanopowder synthesized with H2 into the α-W phase, or to transform the WO3 nanopowder synthesized without H2 into α-W nanopowder without the β-W phase, is indispensable for the preparation of single-phase α-W nanopowder.
To investigate the phase transformation from WO3 into W by thermochemical reduction, samples of the WO3 nanopowder synthesized by RF induction thermal plasma were reduced by annealing at 600-900 °C in an H2 atmosphere for 10 min. It is known that WO3 is reduced to W in an H2 atmosphere which can be explained using Equation (2-5) [2]:
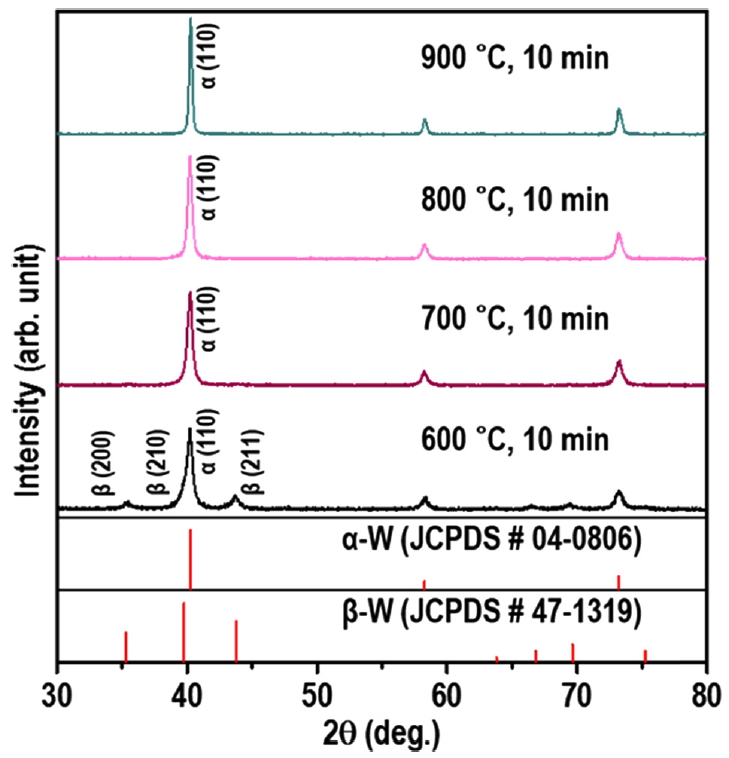
The XRD patterns presented in Fig 5 show that the powders reduced at 700 °C, 800 °C, and 900 °C could be indexed to single-phase α-W and were free of any peaks corresponding to W oxides or β-W. As the temperature for annealing was increased from 700 °C to 900 °C, the main peak of the α-W phase, α-W (110), became sharper, which was attributed to increased crystallinity and particle size due to the higher annealing temperature.
On the other hand, the pattern of the powder reduced at 600 °C corresponded mainly to the α-W phase, but β-W was present as the minor phase. The Rβ-W of the powder calculated with Eq. (1) was 0.22, indicating that the α-W phase accounted for 18% of the W powder.
These XRD results indicate that some fraction of WO3 was transformed into the β-W phase via annealing at 600 °C, while at the higher temperature (700-900 °C) the WO3 was completely transformed into the α-W phase.
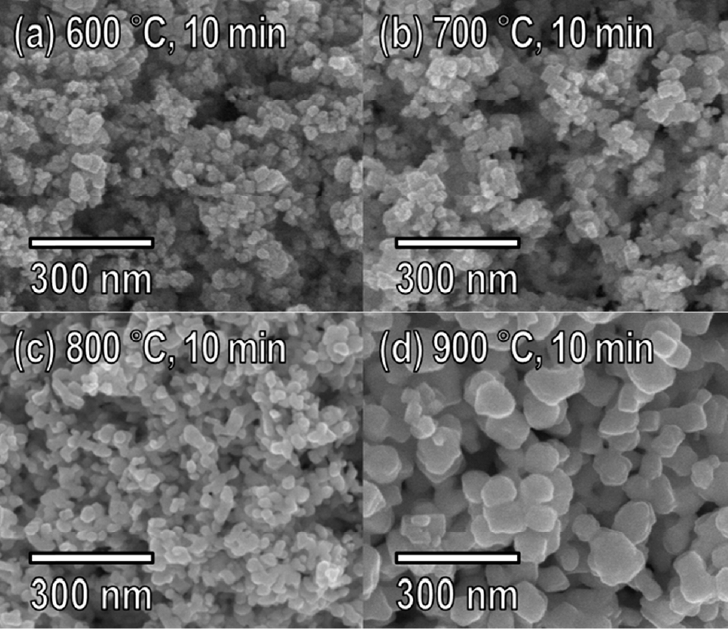
The changes in the morphology of the powders reduced via thermochemical reduction were analyzed with FESEM, as shown in Fig 6. The powders reduced at 600 °C (Fig 6a) and 700 °C (Fig 6b) had particle sizes (d50) of 20.51 nm and 21.16 nm, respectively, which were not significantly different from that of the as-synthesized WO3 nanopowder. However, the powders reduced at 800 °C (Fig 6c) and 900 °C (Fig 6d) contained particles that had agglomerated to form larger particles. The particle size varied dramatically with the annealing temperature, and as a result the XRD peaks sharpened as annealing temperature increased. The atomic composition was also analyzed by EDX before and after the thermochemical reduction. The powder reduced by annealing at 700 °C for 10 min was almost completely transformed from WO3 into W, with the oxygen content from WO3 being reduced from 29.2 wt% to 0.9 wt%.
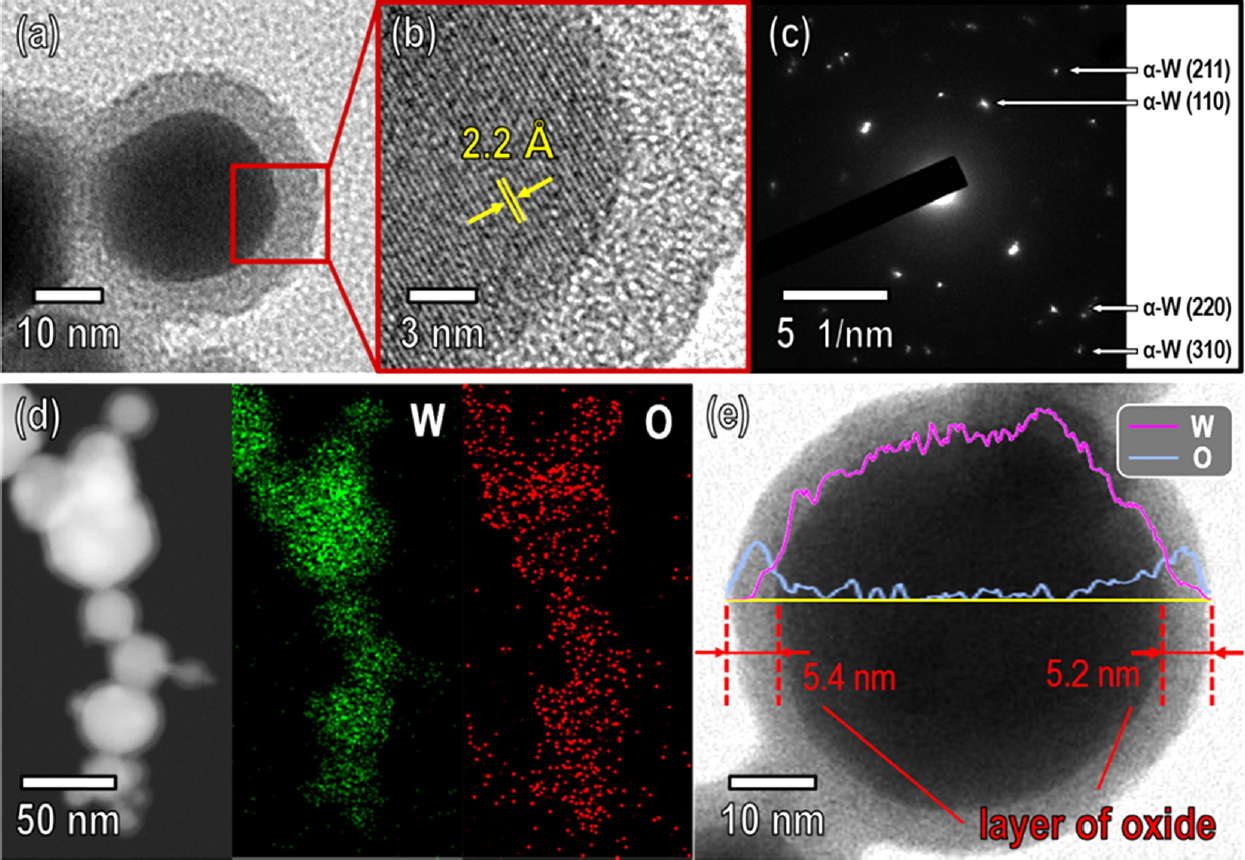
The morphology, crystallinity, and elemental distribution of the W nanopowder reduced by thermochemical reduction at 700 °C for 10 min were further investigated using HRTEM, SAED, elemental mapping, and line scan profile, as shown in Fig 7. Fig 7a shows that the W nanopowder had a spherical shape with a diameter of ~35 nm and was surrounded by an amorphous oxide shell with a thickness of ~5.4 nm, which allowed the W nanopowder to be easily handled in air.
The HRTEM image corresponding to the region marked by the red square in Fig 7a shows that the individual W nanopowder particles were single-crystalline and α-W phase (Fig 4b). The periodic lattice fringes indicated an interatomic plane distance of 2.2 Å, which agreed with the (110) plane of the α-W phase (Fig 7b). The SAED patterns shown in Fig 7c also confirmed that the W nanopowder consisted solely of the bcc structure α-W phase without any β-W or W oxide phase, consistent with the XRD results (Fig 5). The elemental mapping and line scan profile data taken from the reduced W nanopowder, shown in Fig 7d and Fig 7e, verified that the nanopowder consisted only of the element W with an outer oxide shell, indicating that no oxygen atoms diffused into the W nanoparticles during the thermochemical reduction. The line scan profile data also shows that oxygen was present on the surface of the W nanoparticle. This means that the 0.9 wt% oxygen content detected by EDX was not due to the presence of W oxides inside the W nanoparticles, but instead to the amorphous oxide shells covering the surface of the pure metal W nanoparticles.
From Fig 5 and Fig 6, annealing the WO3 nanopowder at 600 °C formed the β-W phase, but not at 700-900 °C. Thus, it is highly feasible that the β-W phase in W nanopowders can be transformed into the α-W phase by annealing at high temperature (700 °C) in an H2 atmosphere. This is in good agreement with the work of É. Hegedüs et al. [32], who reported that the β-W phase was produced by the hydrogen reduction of W oxides at 500-640 °C, and that of Y.G. Shen et al. [31], who reported that the β-W phase was completely transformed into the α-W phase by annealing at 900 K (623 °C). Accordingly, single-phase α-W nanopowder can be obtained by the thermochemical reduction of WO3 nanopowder, and the particle size can be controlled by annealing temperature.
The same experiments were next performed using commercial WO3 powder as a precursor for the synthesis of the single-phase α-W nanopowder for comparison. The results are shown in Fig 8. The results of both the RF induction thermal plasma treatment and the thermochemical reduction using the WO3 precursor were greatly similar to those using the APT precursor. Since APT is generally made from tungsten ore and used as a precursor for WO3, producing W nanopowders from APT in a single step is more economical than doing so from WO3.
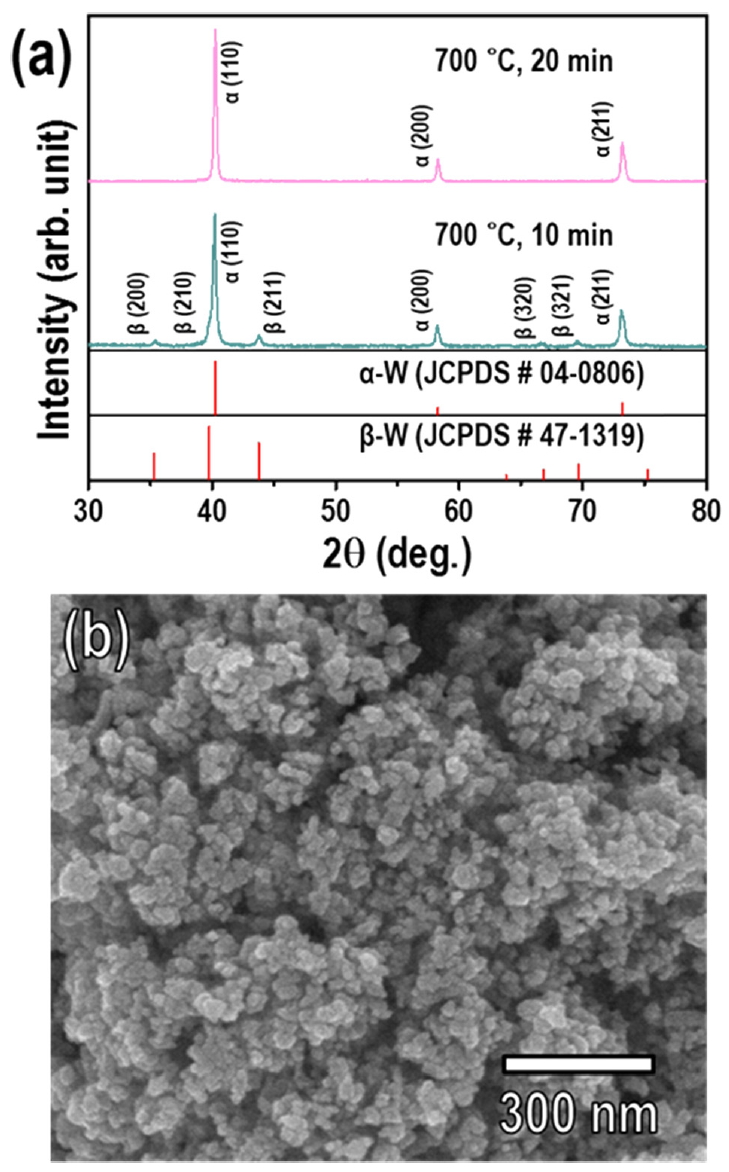
Additionally, to investigate the thermochemical reduction behavior of the β-W to α-W transformation, samples of the mixed-phase α- and β-W nanopowder synthesized by RF induction thermal plasma under Ar sheath gas balanced with 10% H2, corresponding to Fig 3b and Fig 4b, were annealed in an H2 atmosphere (flow rate of H2 = 0.5 L/min) at 700 °C for 10 min and 20 min. When the mixed-phase W nanopowder was reduced by annealing at 700 °C for 10 min, the β-W phase was not completely transformed into the α-W phase, and the sample exhibited a phase mixture. The proportion of β-W phase in the W nanopowder decreased from 26% to 11%, as shown in Fig 9a. When the annealing time was increased to 20 min, the resulting XRD pattern could be indexed to the α-W phase without any β-W phase (Fig 9a), indicating the formation of single-phase α-W nanopowder with a particle size similar to that of the initial mixed-phase W nanopowder (Fig 9b). This means obtaining single-phase α-W nanopowder by the thermochemical reduction of mixed-phase α- and β-W nanopowder requires a remarkably longer annealing time compared with WO3 nanopowder. It also confirms that the β-W phase of W nanopowder can be transformed into the α-W phase by thermochemical reduction without changing the particle size.
These results demonstrate that employing the RF induction thermal plasma process with APT micro powder as the precursor and Ar sheath gas without H2 followed by thermochemical reduction was a more economical and facile method for the synthesis of single-phase α-W nanopowder than the thermal plasma treatment using 10% H2 in the sheath gas, because of its lower energy consumption and shorter annealing time. In other words, to fabricate the single-phase α-W nanopowder, converting WO3 nanopowder synthesized from APT into single-phase α-W was better than transforming mixed-phase α- and β-W nanopowder. Also, compared with the reported synthesis of single-phase α-W nanopowder [3, 6-8, 24], in the present approach particle size can be easily controlled by annealing temperature, the morphology is spherical, and the dispersive condition is equal or superior since there is no remarkable aggregation.
4. CONCLUSIONS
The material process engineering challenges of pure single-phase W nanopowder synthesis has been addressed through a two-stage process. WO3 powder was produced from commercially available APT through RF induction plasma treatment and commercial-grade single-phase α-W nanopowder was then produced by thermochemical reduction at 700 °C for 10 min. Unlike our previously reported W powder synthesis [23], in the current study the WO3 nanopowder synthesized from ATP in the first step was completely transformed into single-phase α-W. Experiments determined that the synthesis of single-phase α-W nanopowder from mixed-phase α- and β-W nanopowder required a longer annealing time (20 min), a relatively energy-intensive process without any remarkable improvement in particle size. These results indicate that the synthesis of single-phase α-W nanopowder using WO3 nanopowder is a more economical and facile method than using mixed-phase α- and β-W nanopowder. The synthetic strategy proposed in this study to produce single-phase α-W nanopowder can also open new avenues for the preparation of other refractory metal nanopowders, such as Ta, Mo, and Zr.