1. INTRODUCTION
Ferritic 9Cr-1Mo steels (ASTM P91) are used in heat exchangers, boilers, and pipes because of their high strength and thermal conductivity, low thermal expansion coefficients, and resistance to creep, thermal fatigue, and oxidation. They form outer Fe-rich oxides such as Fe2O3 and Fe3O4, and inner Cr-rich oxides such as FeCr2O4 or (Fe,Cr)3O4 during high-temperature oxidation in air [1], steam [2], and ultra-supercritical power plant [3]. Although they form protective Cr-rich oxide scales during high-temperature oxidation [4], they become less protective in sulfur-containing environments because sulfides are generally highly nonstoichiometric and have lower melting points than their corresponding oxides [5,6]. Sulfur is usually present in fossil fuels, fluxes, or chemical feedstock. In reducing atmospheres, it exists in the gas stream as H2S(g), which limits operating temperature and efficiency during processing in oil refineries, petrochemical units, coal gasification, natural gas reforming, and energy generation in thermal power plants [7-10]. H2S dissociates into sulfur and hydrogen. Hydrogen atoms dissolve to cause embrittlement, ingress into the scale and metals interstitially, generate lattice point defects and hydrogen clusters, resulting in a significant increase in the corrosion of metals [11].
When corroded in H2/H2S gas at 575-650 ┬░C, 9Cr-1Mo steel formed (FeS, FeCr2S4)-sulfide scales which tended to crack and spall during cooling [12]. In N2/H2S gas at 600-700 ┬░C, it corroded fast and almost linearly, forming outer FeS scales and inner (FeS, FeCr2O4)-mixed scales [13]. Particularly, Fe1-xS grows fast due to its nonstoichiometry (x=0-0.2), and makes the scales highly fragile, non-adherent, and nonprotective [14].
This study aimed to investigate the microstructure and corrosion behavior of hot-dip aluminized 9Cr-1Mo steel in a highly corrosive H2S environment. Aluminizing is widely used on metals in order to protect alloys from oxidation/corrosion [15]. Hot-dip aluminizing of steels leads to the formation of an Al-rich topcoat and the Al-Fe intermetallic layer consisting of Fe2Al5 and FeAl3 [16]. In this study, the corrosion behavior of hot-dip aluminized 9Cr-1Mo steel in N2/H2S gas was investigated at 800-900 ┬░C, because the industrially important H2S gas is particularly destructive at high temperatures. Nitrogen was employed as the balance gas in order to simulate coal gasification systems [8]. This study will be a useful contribution to the development of corrosion-resistant coatings for iron-base alloys.
2. EXPERIMENT DETAILS
A P91 steel plate with a nominal composition of Fe-9Cr-1Mo-0.45Mn-0.4Si-0.2V-0.08Nb-0.1C (wt%) was ground to a 1000 grit SiC finish, immersed in a 10 vol% HCl solution to remove surface oxides, subjected to a liquid flux treatment with 20 vol% (KCl+AlF3 in 4:1 weight ratio) solution, dried, and dipped in Al(l) for 5 min at 800 ┬░C, on top of which a solid flux (KCl+NaCl+AlF3 in 2:2:1 weight ratio) was spread to protect Al(l) from oxidation. After aluminizing, the cleaned samples were corroded at 800 and 900 ┬░C for 50 h under 1 atm of flowing N2/0.1%H2S gas inside a quartz reaction tube furnace. The purity of the N2 and H2S gas was 99.999% and 99.95%, respectively. The samples were inspected using a field-emission scanning electron microscope (SEM) equipped with an energy dispersive spectrometer (EDS), a field-emission electron probe microanalyzer (EPMA), and a high-power X-ray diffractometer (XRD) with Cu-K╬▒ radiation at 40 kV and 150 mA. Microstructures were examined by etching the aluminized coating with KellerŌĆÖs reagent and the steel matrix with VillellaŌĆÖs reagent, together with electron-back scattered diffraction (EBSD). Hereafter, the compositions are denoted in atomic percentages (at%).
3. RESULTS AND DISCUSSION
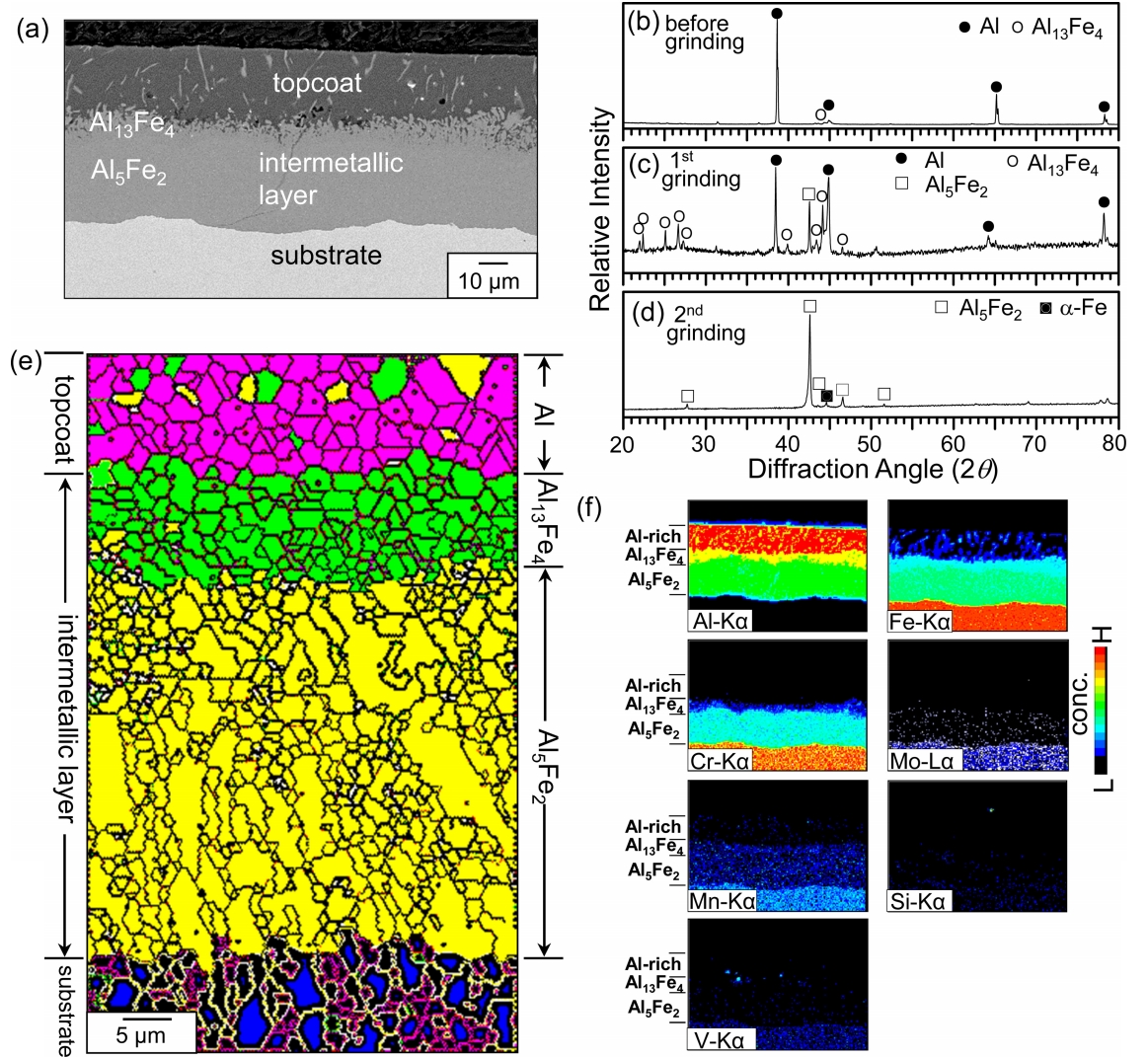
Figure 1 shows the EPMA/XRD/EBSD results of aluminized 9Cr-1Mo steel. The coating consisted of an ~20 ╬╝m thick topcoat and ~50 ╬╝m thick intermetallic layer, which were formed by interdiffusion between the Al(l) and substrate [16] (Fig 1(a)). XRD patterns obtained before and after the successive grinding are shown in Figs 1(b-d). The topcoat primarily consisted of Al with a small amount of Al13Fe4 (Fig 1(b)). When the topcoat was partially ground off, Al13Fe4, Al5Fe2 and Al peaks came out, indicating the presence of Al13Fe4 and Al5Fe2 below the Al-rich topcoat (Fig 1(c)). Further surface grinding revealed Al5Fe2 only (Fig 1(d)). The Powder Diffraction File (PDF) numbers of phases in the Al-Fe binary phase diagram are as follows. Al (85-1327), Al13Fe4 (29-0042), Al5Fe2 (47-1435), AlFe (33-0020), AlFe3 (50-0955), and ╬▒-Fe (06-0696).
According to the concentration gradients of Al and Fe created during hot dipping, an Al-rich topcoat containing a small amount of Al13Fe4 and Al5Fe2 precipitates, an upper Al13Fe4 layer containing a small amount of Al5Fe2 precipitates, and a lower Al5Fe2 layer were formed on the surface (Fig 1(e)). This phase distribution is also recognizable in the Al map shown in Fig 1(f). The Al5Fe2 layer was thick and somewhat columnar, because orthorhombic Al5Fe2 has 30% vacancies along the c-axis, through which the interdiffusion of Al and Fe occurred fast [17,18] (Fig 1(e)). Dissolution of the alloying elements such as Cr, Mo, and Mn in the Al5Fe2 layer flattened the coating/substrate interface and narrowed the Al5Fe2 layer (Figs 1(a, f)). It is noted that, in the case of low-alloyed carbon steels, the coating/substrate interface exhibited a finger- or tongue-like morphology that was oriented along the c-axis of Al5Fe2 layer, and the Al5Fe2 layer was typically thick [19-21].
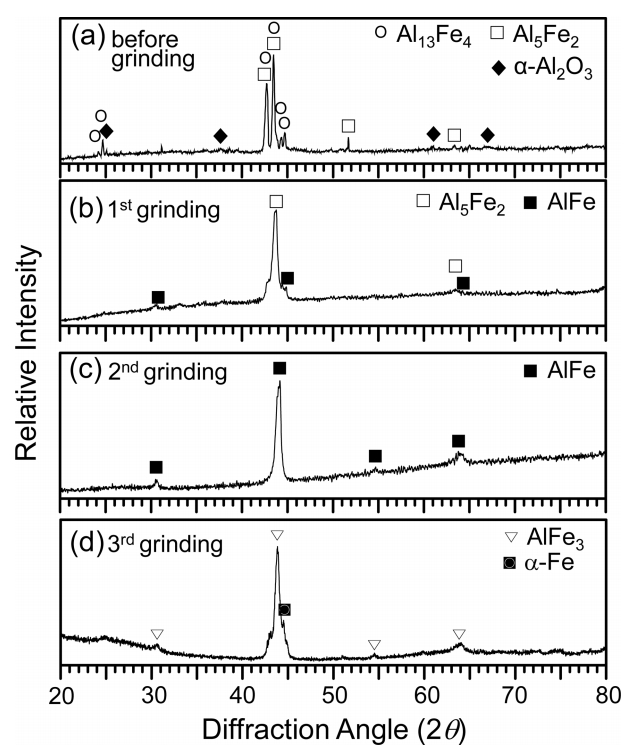
Aluminized 9Cr-1Mo steel was corroded at 800 ┬░C for 50 h, and XRDŌĆÖed while successively grinding the coating surface. In Fig 2(a), the Al13Fe4 and Al5Fe2 peaks are strong, while the ╬▒-Al2O3 peaks are weak. This implies that the Al-rich topcoat transformed to Al13Fe4 and Al5Fe2 through the interdiffusion of Al and Fe, and was also partially oxidized to ╬▒-Al2O3 at the surface. Moving deeper in the coating, the concentration of Al decreased, while that of Fe increased. Hence, strong Al5Fe2 and weak AlFe peaks came out after the first grinding (Fig 2(b)). Only AlFe peaks came out after the second grinding (Fig 2(c)). Strong AlFe3 peaks came out after the third grinding in addition to the ╬▒-Fe substrate peak (Fig 2(d)). In brief, the XRD results indicated the formation of (╬▒-Al2O3 surface scale)/(Al13Fe4, Al5Fe2)-mixed layer)/(AlFe layer)/(AlFe3 layer) on the ╬▒-Fe substrate. This is explained further in Fig 3.
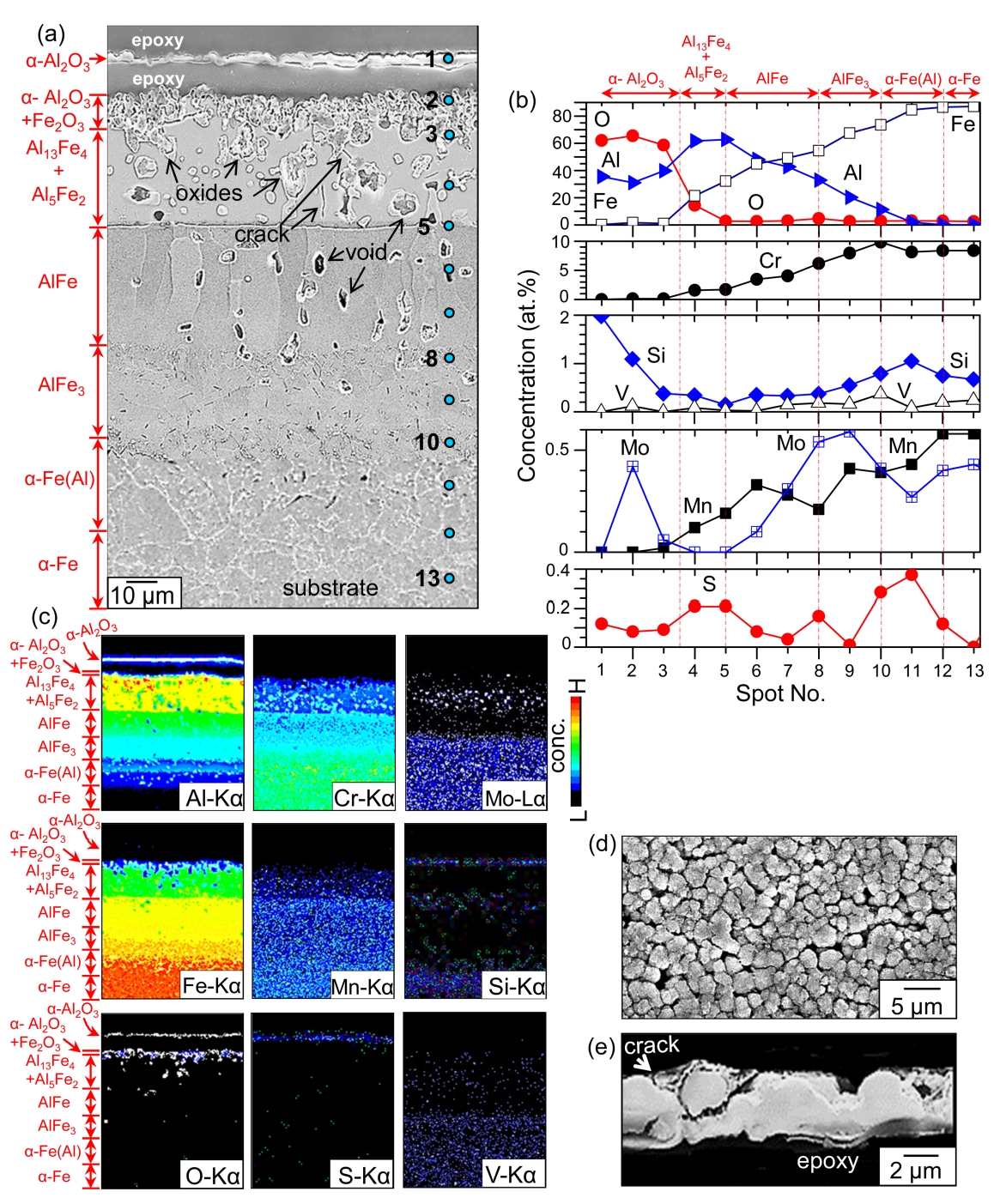
The compositions of spots 1, 2, and 3, denoted in Fig 3(a), were 35.5Al-62.1O-0.3Fe-2Si-0.1S, 31.1Al-65.5O-1.7Fe-0.1Cr-0.4Mn-1.1Si-0.1S, and 39.7Al-58.6O-1.1Fe-0.1Cr-0.4Si-0.1S (at%), respectively, according to FESEM-EDS analysis (Fig 3(b)). This implies that spots 1 and 2-3 correspond to the detached and adherent ╬▒-Al2O3 scale, respectively. A small amount of substrate elements that diffused outwardly during heating presumably dissolved in the ╬▒-Al2O3 scale. The standard Gibbs free energy changes and equilibrium partial pressures for sulfidation/oxidation reaction of Al at 800 ┬░C are as follows [22].
2 Al(s) + 3/2 S2(g) ŌåÆ Al2S3(s); ╬öG┬░ = ŌłÆ619.6 (kJ), Ps2(eq) = 7.8 ├Ś 10-21 (atm)
2 Al(s) + 3/2 O2(g) ŌåÆ ╬▒-Al2O3(s); ╬öG┬░ = ŌłÆ1337.1 (kJ), Po2(eq) = 4 ├Ś 10-44 (atm)
Since Al2O3 is much more stable than Al2S3, the aluminum at spots 1-3 preferentially reacted with impurity oxygen in N2/0.1%H2S gas, resulting in the formation of protective ╬▒-Al2O3 in preference to the less protective Al2S3.
The alumina at spot 1 inevitably spalled for the following reasons. Firstly, growth stress developed due to the dissolution of foreign elements and the phase transformation of Al to ╬▒-Al2O3. Particularly, the sulfur incorporation deteriorated the scale adherence [23]. Secondly, thermal stress was generated due to the mismatch in thermal expansion coefficients between spot 1 and spot 2, whose compositions were different. Thirdly, hydrogen released from H2S gas might have dissolved in ╬▒-Al2O3 and deteriorated the adherence of ╬▒-Al2O3 [11]. Spots 4-5 belonged to the (Al13Fe4, Al5Fe2)-mixed layer (Figs 2(a-b) and 3(a-b)). Spots 5-8 belonged to the AlFe layer (Figs 2(c) and 3(a-b)). The AlFe layer consisted of coarse, columnar grains that aligned along the interdiffusion direction of the Al and substrate elements (Figs 2(b-c) and 3(a-b)). AlFe can dissolve (23.3-55)% Al, according to the Al-Fe phase diagram. Spots 8-10, 10-12, and 12-13 belonged to the AlFe3 layer, the Al-dissolved ╬▒-Fe layer, and the ╬▒-Fe substrate, respectively (Figs 2(d) and 3(a-b)).
Such can also be envisaged in the Al map shown in Fig 3(c). The detached scale at spot 1 consisted of fine, round ╬▒-Al2O3 grains, reflecting its slow growth rate (Fig 3(d)). It cracked easily (Fig 3(e)). All the substrate elements diffused outwardly, while Al, sulfur, and oxygen diffused inwardly, following the concentration gradients (Figs 3(b-c)). This broadened and transformed the original coating. The Kirkendall effect that was produced by the unequal mass flow, as well as the phase transformation of low density, high Al-containing phases to high density, low Al-containing phases, facilitated the formation of voids at spots 3-10 (Fig 3(a)). The densities of Al13Fe4, Al5Fe2, AlFe, and AlFe3 were 3.849. 3.96, 5.67, and 6.57 g┬ĘcmŌłÆ3, respectively. The phase transformation also produced volume shrinkage, developing microcracks to relieve the stress. Cracks were observed between spots 3 and 5, as shown in Fig 3(a).
The generation of voids that can act as stress concentration sites, the mismatch in thermal expansion coefficients among the transformed Al-Fe phases, and the dissolution of sulfur and possibly hydrogen also facilitated the microcracking in the coating. The detachment of the ╬▒-Al2O3 surface layer, and the development of easy-diffusion paths such as voids and microcracks, resulted in the development of internal oxides in the coating, as depicted in the oxygen map shown in Fig 3(c). Like oxygen, sulfur also diffused inwardly through voids, grain boundaries, and microcracks, but to a lesser amount because of its very low solubility in most oxides and metals [24]. This is recognizable from the sulfur map shown in Fig 3(c).
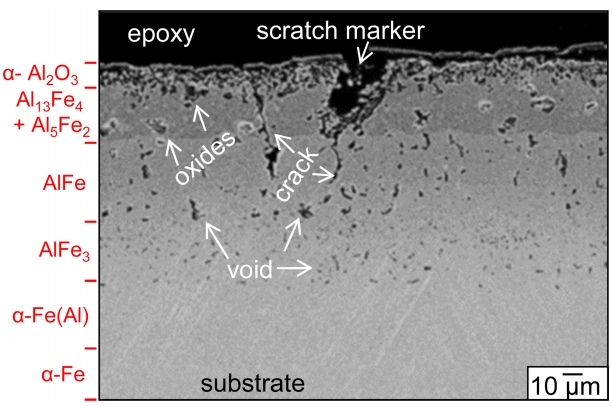
Figure 4 shows an unetched image of the corroded sample. The etched image was shown in Fig 3(a). In Fig 4, a scratch was engraved in the surface prior to corrosion in order to locate the original sample surface. No significant outward diffusion of substrate elements was found to have occurred, although the outward diffusion was confirmed from the formation of internal voids as shown in Fig 3(a). The voids appeared smaller down in the coating. Cracks propagated across the (Al13Fe4, Al5Fe2)-mixed layer and AlFe layer (Fig 4). Among Al-Fe intermetallics, Al13Fe4 and Al5Fe2 are brittle, AlFe is ductile, and Al3Fe is the most ductile [25]. The ╬▒-Al2O3 surface scale was partially detached, below which the adherent ╬▒-Al2O3 layer existed (Fig 4).
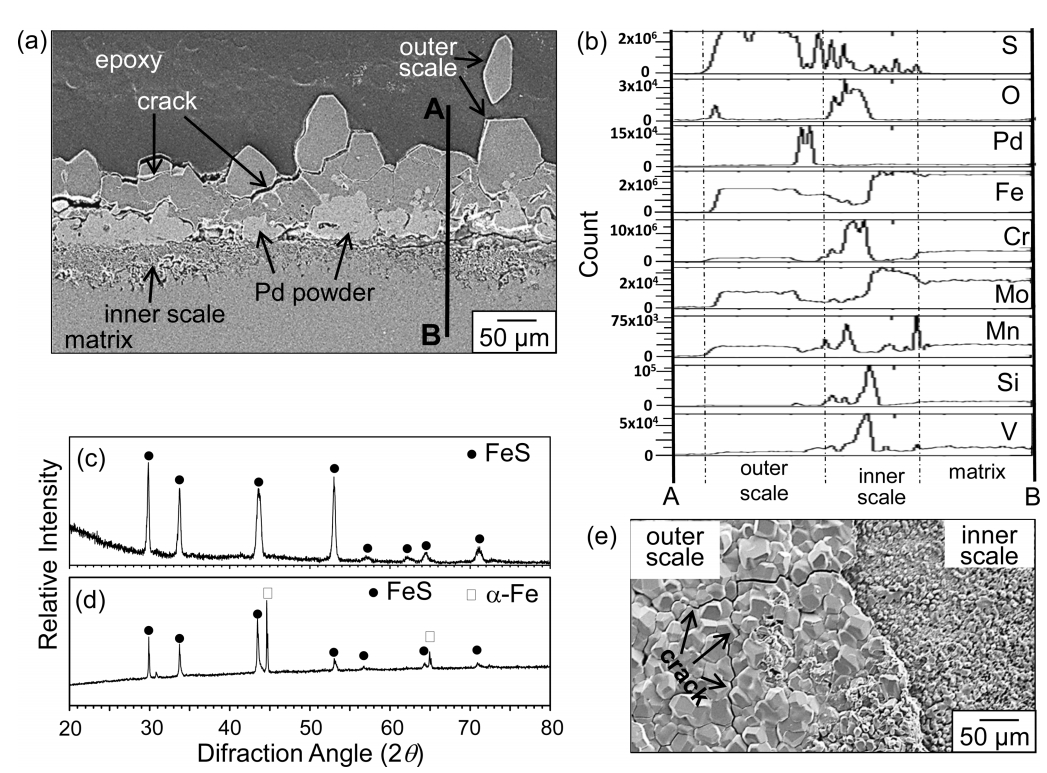
In order to evaluate the effect of aluminizing on corrosion resistance, bare 9Cr-1Mo steel was corroded under the corrosion condition given in Figs 3-4. Prior to corrosion, inert Pd powder (< 1 ╬╝m╬”) was sprayed onto the sample surface for the marker test. The outer and inner scale was about 75-200 and 50 ╬╝m thick, respectively, between which agglomerated Pd powder existed (Fig 5(a)).
Hence, it can be seen that the outer scale grew by the outward diffusion of substrate elements, while the inner scale grew by the inward diffusion of oxygen and sulfur (Fig 5(b)). The XRD analysis indicated that both the outer and inner scales primarily consisted of FeS (Figs 5(c-d)). FeS, whose defect structure is Fe1-xS (x=0-0.2), grows quickly by the outward diffusion of Fe2+ ions [14]. The poor corrosion resistance of the bare 9Cr-1Mo steel was attributed to the formation of nonprotective FeS according to the reaction; Fe+H2SŌåÆFeS+H2. Hydrogen evolution, excessive thickening of the FeS scale, and mismatch in thermal expansion coefficients between the outer and inner scale made the outer scale nonadherent.
The outer FeS scale was coarse and fragile yielding interand trans-granular cracking, while the inner FeS scale was fine and adherent (Fig 5(e)). Small amounts of Cr, Mo, Mn, Si, and V diffused outwardly to dissolve in the outer FeS grains (Fig 5(b)). The consumption of sulfur in the outer FeS scale decreased the sulfur potential and thereby increased the oxygen potential in the inner scale (Fig 5(b)). However, the amount of oxygen in N2/0.1%H2S gas was at the impurity level, so the inner scale primarily consisted of FeS (Fig 5(d)). In contrast, aluminized 9Cr-1Mo steel displayed good corrosion resistance by forming highly protective, stable ╬▒-Al2O3 through the reaction of Al with impurity oxygen in N2/0.1%H2S gas, as outlined in Figs 2-4.
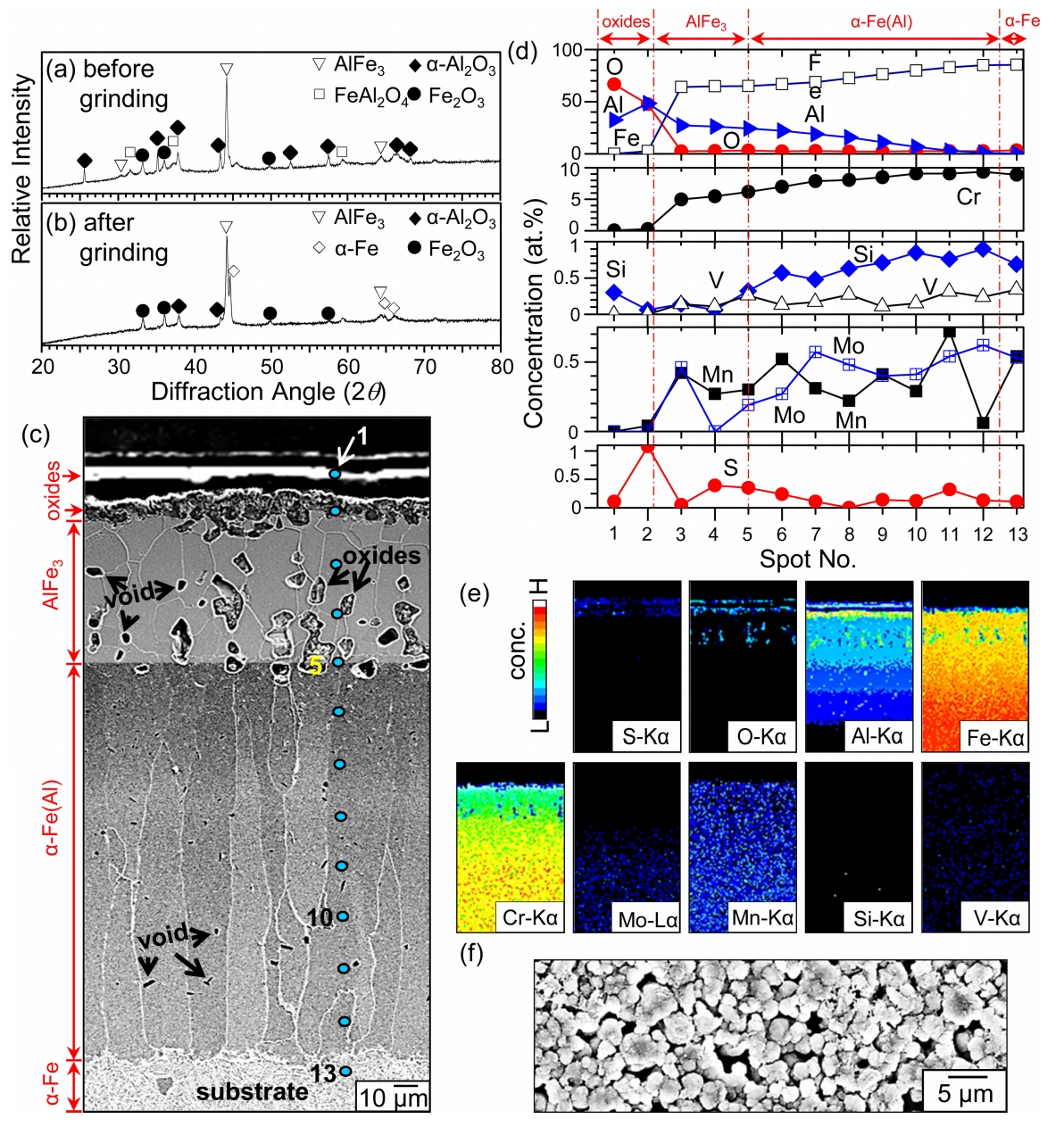
Figure 6 shows XRD/SEM/EDS/EPMA results for the 9Cr-1Mo steel after corrosion at 900 ┬░C for 50 h. Figure 6(a) indicates the formation of ╬▒-Al2O3, Fe2O3, and FeAl2O4 around the AlFe3 coating. This suggests that aluminum in the original Al-rich topcoat oxidized to ╬▒-Al2O3. At the same time, iron that diffused outward across the original Al-rich topcoat according to the concentration gradient oxidized to Fe2O3, and some of Fe2O3 was further transformed to the more stable FeAl2O4 spinel. When the surface was ground off, ╬▒-Fe newly came out besides ╬▒-Al2O3, Fe2O3, and AlFe3 (Fig 6(b)). This indicates the presence of ╬▒-Fe underneath the AlFe3 coating containing some oxides. The above oxides and coating phases are illustrated in Fig 6(c).
Spot 1 in Fig 6(c) indicates ~5 ╬╝m-thick, nonadherent ╬▒-Al2O3 scale with a composition of 32.5Al-66.6O-0.1S-0.1Cr-0.2Mo-0.5Si (%), according to the EDS spot analysis (Fig 6(d)). The dissolution of foreign elements, including sulfur, favored spallation of the ╬▒-Al2O3 surface scale. Spot 2 was located at the (adherent scale)/(AlFe3 coating) interface. The adherent scale was ~10 ╬╝m-thick, and mostly consisted of ╬▒-Al2O3. Fe2O3 and FeAl2O4 tended to exist underneath the adherent or nonadherent ╬▒-Al2O3ŌĆōrich surface scale, as can be noted from the O-Al-Fe maps shown in Fig 6(e).
Spots 2-5 indicate a ~40 ╬╝m-thick AlFe3 layer containing some oxides and voids (Fig 6(c)). Their average composition was 26.8Al-64.4Fe-2.6O-0.2S-5.2Cr-0.2Mo-0.4Mn-0.1Si-0.1V (%), implying the incorporation of oxygen, sulfur, and alloying elements in coarse AlFe3 grains (Figs 6(c-e)). Spots 5-12 indicate a ~110 ╬╝m-thick Fe(Al) layer. Their average composition was 76Al-11.2Al-8.4Cr-0.5Mo-0.4Mn-0.7Si-0.2V-2.5O-0.1S, indicating that coarse, columnar ╬▒-Fe grains were dissolved with a large amount of Al plus a small amount of alloying elements, oxygen, and sulfur (Figs 6(c-e)). The oxygen map shown in Fig 6(e) indicates scattered internal oxides at spots 2-5. Dark dots at spots 5-12 were voids that formed primarily due to the Kirkendall effect (Fig 6(c)). The sulfur map shown in Fig 6(e) indicates the presence of sulfur sporadically distributed around the surface.
Oxygen and sulfur can ingress rather easily through Kirkendall voids, and imperfect oxide scale. However, the sulfur concentration was less than 1% at spots 1-13, because the solubility of sulfur in most oxides and metals was very low [24] (Fig 6(d)). The detached scale at spot 1 primarily consisted of fine, round ╬▒-Al2O3 grains, reflecting the slow growth rate of alumina (Fig 6(f)). This is also displayed in Fig 3(d). Despite the detachment and imperfection of the ╬▒-Al2O3ŌĆōrich scale, the aluminized coating effectively resisted the harsh H2S-gas corrosion.
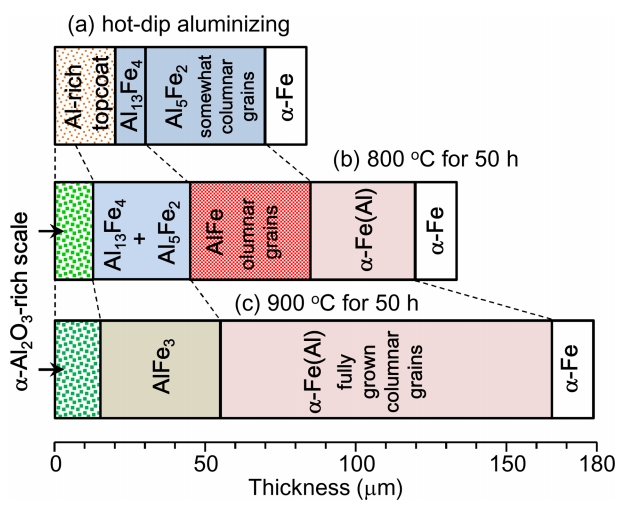
Results obtained from Figs 2-4 and 6 can be summarized as follows. Corrosion at 800 ┬░C for 50 h produced an ╬▒-Al2O3 layer with a small amount of Fe2O3, an (Al13Fe4, Al5Fe2)-mixed layer, an AlFe layer consisting of columnar grains aligned along the interdiffusion direction, and an ╬▒-Fe(Al) layer. Corrosion at 900 ┬░C for 50 h produced the ╬▒-Al2O3 and AlFe3 layers with some Fe2O3 and FeAl2O4, and the ╬▒-Fe(Al) layer consisting of fully grown columnar grains. This transformation of the Al-Fe phases in the coating at 800-900 ┬░C originated from the interdiffusion of Al and Fe in the Al/Fe diffusion couple. For example, the interdiffusion coefficient of Fe and Al in the iron aluminide was determined to be 5.93 ├Ś 10-16 and 2.92 ├Ś 10-14 m2/s at 550 and 640 ┬░C, respectively [26]. Likewise, increasing the corrosion temperature from 800 to 900 ┬░C transformed (high Al)-Fe phases to (low Al)-Fe phases, and drastically broadened the coating, as schematically illustrated in Fig 7.
4. CONCLUSIONS
ASTM P91 steel was subjected to a hot-dip aluminized coating, which consisted of an Al-rich topcoat with a small amount of Al13Fe4 and Al5Fe2 precipitates, an Al13Fe4 layer having a small amount of Al5Fe2 precipitates, and an Al5Fe2 layer with dissolved substrate elements. Aluminizing effectively improved corrosion resistance by forming Al2O3-rich surface scales through the preferential reaction of Al with impurity oxygen in corrosive N2/0.1%H2S gas. When a bare sample was exposed, a nonprotective, thick, bilayered FeS scale formed. In the aluminized samples, substrate elements diffused outwardly, while Al, sulfur, and oxygen diffused inwardly, which broadened and transformed the Al-Fe phases during corrosion. This was accompanied by the formation of internal oxides, voids, and microcracks in the coating.