1. INTRODUCTION
Inorganic acids such as hydrochloric, nitric, and sulfuric acid have been widely used in hydrometallurgical processes for the dissolution of desired valuable metals from ores or secondary resources. The nature of these inorganic acids affects the subsequent processes used to separate the metal ions from the leaching solution. And because the effluents from these separation steps contain these inorganic acids, their disposal is of environmental concern [1,2]. A number of methods are available to treat effluents containing inorganic acids, including neutralization, diffusion dialysis, membrane process, precipitation, solvent extraction, and ion exchange processes [3-5].
Among these methods, solvent extraction is considered to be one of the most effective for extracting inorganic acids from effluents. During solvent extraction, the reaction between the extractants and the protons (hydrogen ions) of the acid might be responsible for the extraction of these acids. Tertiary amines such as Alamine 308 (tri-isooctyl amine), Alamine 336 (tri-C8-10 alkyl amines), TDA (tri-ndodecyl amine), TEHA (tris 2-ethylhexyl amine), TIOA (triisooctyl amine), and TOA (trioctylamine) are generally used for the selective extraction of hydrogen ions. Most of the extraction reactions follow the protonation mechanism [6-8]. In another approach, neutral extractants such as TBP (tributyl phosphate), MDP (methyl diphenyl phosphate), and Cyanex 923 (a mixture of four trialkylphosphine oxides) can also extract hydrogen ions efficiently via the solvating mechanism [9,10]. While ammonium salts like Aliquat 336 or cationic extractants like Cyanex 272 cannot by themselves extract inorganic acids, binary mixtures consisting of Aliquat 336 and Cyanex 272 or their mixtures with other extractants has a significant effect on the extraction of hydrogen ions in inorganic acids [11,12]. It has also been reported that some mixtures fail to enhance the extraction of hydrogen ions [6,13]. Therefore, the hydrogen ion extraction behavior of binary mixed systems deserves investigation.
Ionic liquids, known as green chemicals, have been extensively employed in many fields and in many processes, including catalysis, electrochemistry, organic synthesis, and the separation and recovery of metals [14-16]. One of the common ionic liquids can be prepared in the laboratory by mixing Aliquat 336 and organophosphorus acids like Cyanex 272. Many studies have demonstrated the excellent efficiency of ionic liquids derived from Aliquat 336 and Cyanex 272 in solvent extraction systems [17-19]. However, very little research has investigated the use of ionic liquids to extract hydrogen ions. It has been observed that during the extraction of metal ions by ionic liquids, the equilibrium pH of the aqueous phase can be higher than the initial pH in the range from 3 to 6 [17,18,20]. Since metal ions can be precipitated when the solution pH is higher than a certain threshold, control of solution pH is very important in the continuous operation of solvent extraction.
In synergistic solvent extraction, the distribution ratios of the mixtures are higher than the sum of the distribution ratios of the individual components in the mixtures [21]. To the best of our knowledge, the mechanism of hydrogen ion extraction by ionic liquids has not yet been identified. Therefore, in this study, ionic liquids consisting of a mixture of Aliquat 336 and Cyanex 272 were employed to investigate the extraction of hydrogen ions. The mechanism of hydrogen ion extraction by ionic liquids was identified using the slope analysis method, and varying some parameters including solution pH, the concentration of the ionic liquid and chloride ions. The motivation of this work was to identify the change in solution pH during extraction by ionic liquid. Our results can explain the change in solution pH during the extraction of metal ions from a weak acidic solution by an ionic liquid prepared from Aliquat 336 and Cyanex 272. This result also shows that the ionic liquid R4NA has the potential to extract acids from aqueous solutions, and thus this work can be applied to recover inorganic acids by extraction with R4NA.
2. EXPERIMENTAL
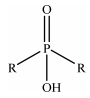
The acidity of the solution was adjusted by adding doubly distilled water to concentrated HCl (Daejung Co., 35%). NaCl (Tedia Company, Inc., 99%) was employed to fix the ionic strength of the solution at a constant value. The extractants Alamine 336 (BASF Co., 95%), Aliquat 336 (NMethyl-N, N, N-trioctylammonium chloride, BASF Co., 93%), Cyanex 272 (bis(2,4,4-trimethylpentyl)phosphinic acid, Cytec Inc., 85%) were used without employing any purification. The structures and properties of these extractants are shown in Table 1. An ionic liquid (R4NA) was prepared by mixing an equimolar concentration of Aliquat 336 (R4NCl) and Cyanex 272 (HA) according to the method reported in the literature [11]. Kerosene (Samchun Pure Chemical Co.) was used as a diluent and all the other chemicals used were of analytical grade.
The extraction experiments were conducted by mixing equal volumes of aqueous and organic phases in a screwed cap bottle for 30 min using a Burrell wrist action shaker (model 75, USA) at ambient temperature. The aqueous phase was separated using a separation funnel after mixing the two phases for 30 min. The initial and equilibrium pH values of the aqueous solution were measured three times using an Orion Star thermal scientific pH meter (model A221, USA). Additionally, the concentration of hydrogen ions in the aqueous phase was also measured before and after extraction by volumetric titration method [22]. When the solution pH was higher than 4, it was difficult to determine the concentration of hydrogen ions by titration method, owing to the negligible concentration of hydrogen ions. Since the ionic strength of the solution was controlled to 0.1 M, the change in the concentration of hydrogen ions during the extraction was determined from the solution pH values when the solution pH was higher than 4. The concentration of hydrogen ions in the organic phase was calculated by mass balance. The distribution ratio (D) was calculated as the concentration of hydrogen ions in the organic phase to that in the aqueous phase at equilibrium. An ultraviolet visible (UV-1800, Shimadzu, Japan) and Fourier transform infrared (FTIR-Vertex 80 V, Bruker, Germany) spectrometers were employed to analyze the spectra of reagents before and after extraction.
3. RESULTS AND DISCUSSION
3.1. Effect of pH on the extraction of hydrogen ions
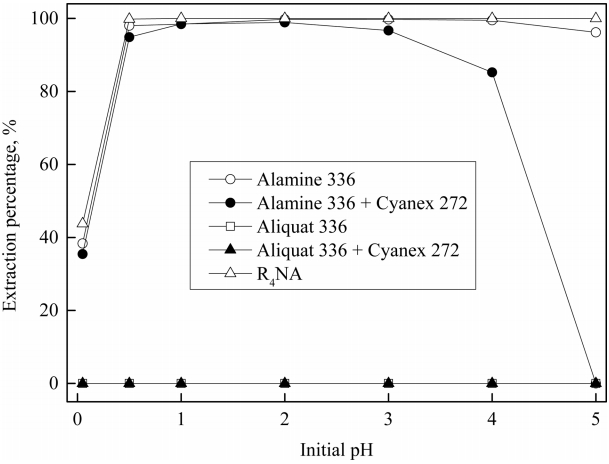
Five kinds of extractants (single Alamine 336 and Aliquat 336, the mixture of Alamine 336 and Aliquat 336 with Cyanex 272 and ionic liquid R4NA) were employed to investigate their hydrogen ion extraction behavior from chloride solutions with an initial pH range of 0.05 to 5. In these experiments, the concentration of each extractant was kept at 0.5 M and the phase ratio of O/A was fixed at unity. Among the 5 kinds of extractants, single Aliquat 336 and its mixture with Cyanex 272 did not extract any hydrogen ions in the experimental range (see Figure 1). The extraction percentage of hydrogen ions by Aliquat 336 and the mixture of Aliquat 336 and Cyanex 272 was nil due to their natural structural properties, that is, Aliquat 336 is an ammonium salt and Cyanex 272 is an acidic extractant.
The extraction percentage of hydrogen ions by Alamine 336 and R4NA were similar to each other. Both increased as the initial pH was increased from 0.05 to 0.5, and then remained almost constant with further increase up to 5. A small decrease in the extraction percentage of hydrogen ions by Alamine 336 was observed when the initial pH was higher than 4, which might be ascribed to the protonation reaction of Alamine 336 [12,23]. The extraction reaction of hydrogen ions by Alamine 336 (R3N) and R4NA from chloride solution can be represented as Eqs. (1) and (2-3), respectively [11,24].
In the mixture of Alamine 336 and Cyanex 272, hydrogen ion extraction efficiency increased as the initial pH increased up to 2, and then decreased with a further increase in initial pH of the solution. At an initial pH of 5, hydrogen ions cannot be extracted by the binary mixture because some competitive reactions occur during the extraction. Before the extraction of hydrogen ions, an amine salt (R3NHA) may form through the ion-pair formation reaction when Alamine 336 is mixed with Cyanex 272, as represented by Eq. (4). Then, hydrogen ions can be extracted by the displacement reaction of the amine salt, as represented in Eq. (5). The other displacement reaction can occur between the anionic species in the organic mixture and chloride ions (Eq. (6)) [12,23]. Eq. (7) indicates that the interaction reaction between Cyanex 272 and the protonated amine results in the release of free acid from the organic phase and thus reduces the extraction of hydrogen ions. Therefore, the extraction percentage of hydrogen ions by the mixture of Alamine 336 and Cyanex 272 was negligible when the initial pH was 5.
The results indicate that ion-pair formation, the protonation of amine, anion exchange, and displacement reactions can occur when an amine interacts with an organophosphorus acid [23]. Moreover, the extraction behavior of hydrogen ions by the above-mentioned extractants depends on the solution pH. Table 2 shows the equilibrium pH values of the solution with these extractants. The equilibrium pH increased as the initial pH increased. This implies that hydrogen ions were well extracted into the organic phase by employing Alamine 336, the mixture of Alamine 336 and Cyanex 272, and R4NA. Comparing these three types of extractants, the extraction efficiency and the variation in the equilibrium pH by R4NA was higher than that of Alamine 336 and its mixture with Cyanex 272. Therefore, the R4NA extractant was chosen for further experiments.
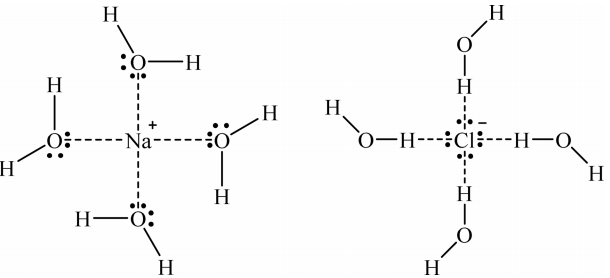
The formation of an emulsion and difficulty in phase separation were observed when the solution pH was higher than 0.5 while employing 0.5 M of each extractant. To circumvent these phenomena, sodium chloride was added to the solution. In addition, the solubility of some extractants in water implies that there is an interaction between the extractants and water molecules that may interfere the extraction of hydrogen ions (see Table 1). Figure 2 shows that the solvation of cations (Na+) and anions (Cl−) by water molecules leads to aqueous stability. That is, the cations and anions can interact with water molecules by electrostatic interactions or the formation of hydrogen bonding, which improves phase separation [25].
3.2. Mechanism of hydrogen ion extraction
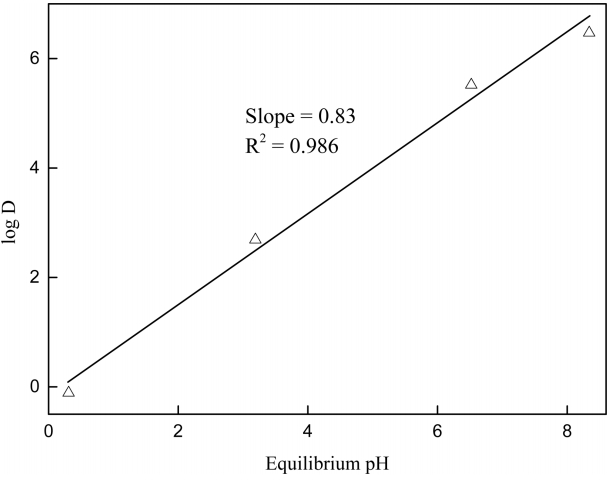
Since few studies have been conducted to analyze the hydrogen ion extraction behavior of the ionic liquid R4NA, the extraction reaction of hydrogen ions by R4NA was investigated here by employing the slope analysis method. In order to examine the effect of solution pH on the extraction of hydrogen ions, 0.1 M NaCl was added to the solution and the initial pH was varied from 0.05 to 2. The concentration of R4NA was fixed at 1 M. In all the experiments, solvent extraction was carried out at an O/A phase ratio of unity. The plot of log D versus equilibrium pH for hydrogen ion extraction is shown in Figure 3. The slope value of the straight line was 0.83, indicating that one mole of hydrogen ions takes part in the extraction. The results agree well with Eqs (2) and (3).
In order to investigate the effect of extractant concentration on the extraction of hydrogen ions from chloride solution, the concentration of R4NA was varied from 0.1 to 0.5 M. In these experiments, the initial pH of the solution was fixed at 2 and sodium chloride concentration was controlled at 0.1 M. Figure 4 shows the plot of log D versus log[R4NA] for the extraction data. The value of the slope was 1.96, indicating that two moles of R4NA were associated with one mole of hydrogen ions in the reaction.
Generally, the molar ratio of hydrogen to chloride ions might be equal for reactions occurring in the aqueous solution. Since one mole of hydrogen ions is extracted by two moles of R4NA, it can be predicted that one mole of chloride ions will be associated with one mole of hydrogen ions during the extraction. In order to verify this, the effect of chloride ion concentration on the extraction of hydrogen ions with R4NA was investigated, by controlling the concentration of sodium chloride from 0.01 to 0.5 M.
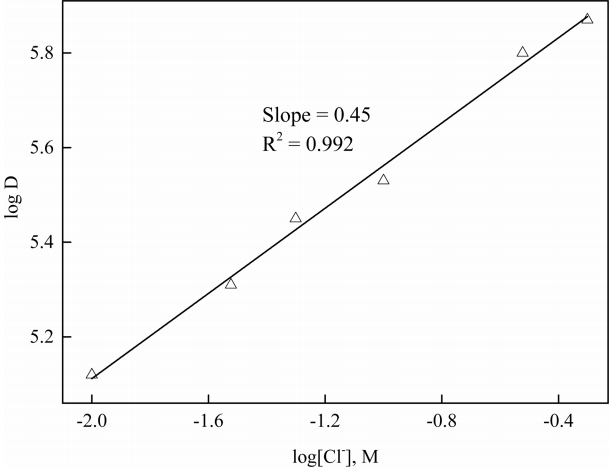
In these experiments, the concentration of R4NA was kept at 0.1 M and the initial pH of the aqueous solution was adjusted to 2. The plot of log D versus log[Cl−] in Figure 5 gives a straight line with a slope value lower than one, implying that the chloride ions take part in the reaction. Since one mole of hydrogen ions was extracted into the organic phase, it should be accompanied by one mole of chloride ions. Among the added chloride salts, some of the sodium chloride can interact with water molecules (see Fig 2), while the rest participates during the extraction by R4NA.
Considering the dependence of log D on the equilibrium pH, the concentration of sodium chloride and R4NA, the overall extraction reaction of hydrogen ions in chloride solution with ionic liquid (R4NA) can be described by Eq. (8). Eq. (8) shows that the extraction of hydrogen and chloride ions with R4NA is similar to that of neutral extractants. Therefore, some further experiments should be performed to verify the species extracted by R4NA ionic liquid.
3.3. Analysis of the species extracted by ionic liquids (R4NA)
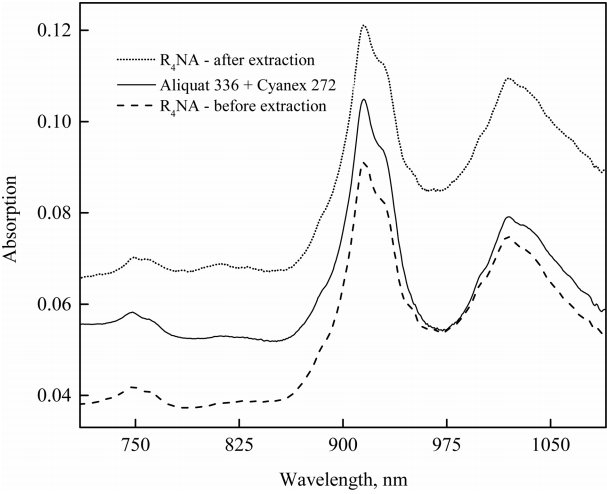
To investigate the species extracted by R4NA after the extraction of hydrogen ions in chloride solution, UV-Vis absorption and FT-IR spectra of the extractant were recorded. The samples were prepared by fixing the concentration of sodium chloride at 0.1 M and adjusting the initial pH of chloride solution at 2. The aqueous phase was contacted with the organic phase at an O/A phase ratio of unity. The concentration of R4NA was 1 M and a mixture of 0.5 M Aliquat 336 and 0.5 M Cyanex 272 in kerosene was also prepared for comparison with R4NA before extraction. The obtained results are shown in Figure 6. R4NA before extraction showed lower intensity than the mixture of Aliquat 336 and Cyanex 272, indicating that the ionic liquid was successfully prepared. Based on the results, ionic liquid R4NA after extraction had the strongest intensity, and the intensity was nearly two times as strong as the intensity of R4NA before extraction. It can be said that hydrogen and chloride ions were transferred from the aqueous to the organic phase.
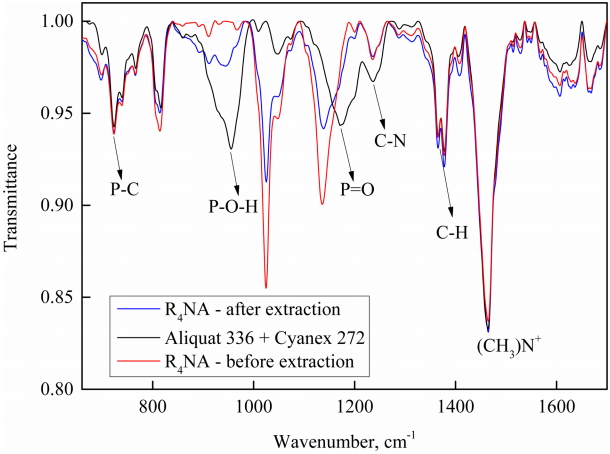
Figure 7 presents the FT-IR spectra of the extractants before and after the extraction of hydrogen ions. Most of the significant changes occurred in the range of 650-1700 cm-1. The spectra in Fig 7 showed the frequencies at 723, 955, 1172, 1236, 1365-1378, and 1465 cm-1, corresponding to the stretching of P-C, P-O-H, P=O, C-N, C-H, and (CH3)N+ groups, respectively, which is considered to be an important vibrational characteristic of Cyanex 272 and Aliquat 336.
There were some changes in the vibration frequency between R4NA and a mixture of Aliquat 336 and 272 Cyanex. Namely, the medium frequencies at 955 cm-1 of PO-H group and 1172 cm-1 of P=O group in the mixture shifted to low-intensity bands at 911 cm-1 and a strong frequency at 1136 cm-1 in R4NA, respectively. This showed that the prepared ionic liquid R4NA had different characteristics than the original precursors. Comparing the FT-IR spectra of R4NA before and after hydrogen ion extraction, no new peaks appeared in the experimental ranges. These results indicate that the ingredient R4NA participated in the extraction of hydrogen ions. Especially at frequencies of 1025 and 1136 cm-1, the intensity of R4NA after extraction was reduced to almost half compared to that before extraction, indicating the strong interaction between hydrogen ions and R4NA.
These obtained results suggest that the hydrogen ions extraction reaction by R4NA in chloride solution followed Eq. (8). It might be said that a solvation reaction was responsible for the extraction. The equilibrium constant (Ke) of Eq. (8) can be given as Eq. (9). Taking the logarithm of Eq. (9) and rearranging, leads to Eq. (11), indicating the dependence of the distribution ratio on the equilibrium constant, the concentration of the ionic liquid and chloride ions.
3.4. Comparison of the extraction of hydrogen ions by mixture extractants
As seen in Fig 1, the combination of Alamine 336 and Cyanex 272 exhibited a synergistic effect on the extraction of hydrogen ions in the initial pH range from 0.5 to 3, which agrees well with the previous work [12]. Meanwhile, the nature of the interaction between Aliquat 336 and Cyanex 272 does not affect the synergism in the extraction reaction. Since the ionic liquid R4NA was prepared by mixing Aliquat 336 and Cyanex 272, the hydrogen ions extraction behavior of R4NA is of great significance. A comparison of the extraction of hydrogen ions by several binary mixtures is given in Table 3. Based on Table 3, there were some differences between this work and previous work in the results for equilibrium pH and the percentages of hydrogen ion extraction by the mixture of Alamine 336 and Cyanex 272 [12]. For instance, the extraction percentage of hydrogen ions by the mixture of Alamine 336 and Cyanex 272 in this system (98.5%) was higher than that in the previous work (92.8%). This may be attributed to the presence of sodium chloride in the solution, which affects the extraction.
Moreover, the ionic liquid R4NA prepared from Cyanex 272 and Aliquat 336 showed better efficiency at extracting hydrogen ions. The change in equilibrium pH value after hydrogen ion extraction by R4NA was three times higher than by the mixture of Alamine 336 and Cyanex 272 in the previous work. R4NA exhibited superior extraction characteristics compared to the other mixture extractants in terms of a change in solution pH.
It can be said that the selective extraction of hydrogen ions by this kind of ionic liquid greatly affects the control of solution pH during the extraction of metals from weak acidic solution. The formation of precipitates can result in crud formation which militates against a continuous solvent extraction operation. Therefore, the selection of an appropriate concentration of ionic liquid is very important, to control solution pH within the stable region of the target metal ions. In order to accomplish this, it is necessary to develop a thermodynamic model of the solvent extraction of metals from weak acidic solution by an ionic liquid.
4. CONCLUSIONS
The solvent extraction of hydrogen ions from chloride solution was investigated using Alamine 336, Aliquat 336 and its mixture with Cyanex 272, and ionic liquids (R4NA). The extractants’ hydrogen ion extraction behavior was found to depend strongly on the solution pH. When the initial pH was increased, the equilibrium pH increased when using Alamine 336, the mixture of Alamine 336 and Cyanex 272, and R4NA. Among these three extractants, R4NA showed the best performance for the extraction of hydrogen ions when the initial pH range was from 0.05 to 5. The formation of an emulsion and difficulty in phase separation were observed, and suppressed by adding sodium chloride. UV-Vis and FTIR spectra analyses verified the extracted species after hydrogen ion extraction by R4NA. The solvent extraction reaction of hydrogen ions by R4NA was determined by applying a slope analysis method to the extraction data. Further work is necessary to develop a thermodynamic model which considers the change in solution pH during the extraction of metal ions in weak acidic solution by an ionic liquid.